Dosing & Uses
Dosage Forms & Strengths
tablet, immediate-release (Keppra, generic)
- 250mg
- 500mg
- 750mg
- 1000mg
3-D tablet, immediate-release (Spritam)
- 250mg
- 500mg
- 750mg
- 1000mg
tablet, extended-release
- 500mg (Keppra XR)
- 750mg (Keppra XR)
- 1000mg (Elepsia XR)
- 1500mg (Elepsia XR)
oral solution (Keppra, generic)
- 100mg/mL
injectable solution
- 5mg/mL
- 10mg/mL
- 15mg/mL
- 100mg/mL
Myoclonic Seizures
Immediate-release (Keppra, Spritam): 500 mg IV/PO q12hr; may increase q2week by 500 mg/dose to recommended dose of 1500 mg q12hr
Effectiveness of doses >3000 mg/day has not been adequately studied
Partial Onset Seizure
Indicated for treatment of partial-onset seizures
Need for oral loading dose not established
Immediate-release (Keppra, Spritam): 500 mg PO q12hr; may increase q2week by 500 mg/dose; not to exceed 3000 mg/day
Extended-release (Keppra XR or Elepsia XR): 1000 mg PO qDay; may increase q2week by 1000 mg/day; not to exceed 3000 mg/day
IV: 500 mg q12hr; may increase q2week by 500 mg/dose; not to exceed 3000 mg/day
Primary Generalized Tonic-Clonic Seizures
Need for oral loading dose not established
Immediate-release (Keppra, Spritam): 500 mg IV/PO q12hr; may increase q2week by 500 mg/dose to recommended dose of 1500 mg q12hr
Effectiveness of doses >3000 mg/day has not been adequately studied
Dosage Modifications
Renal impairment
-
Immediate release and IV formulations
- CrCl >80 mL/min/1.73 m²: Dose adjustment not required
- CrCl 50-80 mL/min/1.73 m²: 500-1000 mg PO q12hr
- CrCl 30-50 mL/min/1.73 m²: 250-750 mg PO q12hr
- CrCl <30 mL/min/1.73 m²: 250-500 mg PO q12hr
- Dialysis (conventional): 500-1000 mg PO qDay, THEN 250-500 mg supplemental dose after dialysis
-
Extended release tablets (Keppra XR)
- CrCl >80 mL/min/1.73 m²: Dose adjustment not required
- CrCl 50-80 mL/min/1.73 m²: 1000-2000 mg PO q24hr
- CrCl 30-50 mL/min/1.73 m²: 500-1500 mg PO q24hr
- CrCl <30 mL/min/1.73 m²: 500-1000 mg PO q24hr
- End-stage renal disease requiring hemodialysis: Immediate release formulation recommended
-
Extended release tablets (Elepsia XR)
- CrCl >80 mL/min/1.73 m²: Dose adjustment not required
- CrCl 50-80 mL/min/1.73 m²: 1000-2000 mg PO q24hr
- CrCl <50 mL/min/1.73 m²: Not recommended
- End-stage renal disease requiring hemodialysis: Immediate release formulation recommended
Dosing Considerations
Avoid abrupt withdrawal to reduce the risk of increased seizure frequency and status epilepticus
Dosage Forms & Strengths
tablet, immediate-release (Keppra, generic)
- 250mg
- 500mg
- 750mg
- 1000mg
disintegrating tablet, immediate-release (Spritam)
- 250mg
- 500mg
- 750mg
- 1000mg
tablet, extended-release
- 500mg (Keppra XR)
- 750mg (Keppra XR)
- 1000mg (Elepsia XR)
- 1500mg (Elepsia XR)
oral solution (Keppra, generic)
- 100mg/mL
injectable solution
- 5mg/mL
- 10mg/mL
- 15mg/mL
- 100mg/mL
Partial Onset Seizures
Immediate-release tablets (Keppra)
- Indicated for monotherapy or adjunctive treatment of partial-onset seizures in patients aged ≥1 month
- <1 month: Safety and efficacy not established
- 1-6 months: 7 mg/kg PO q12hr; increase by increments of 7 mg/kg q12hr q2weeks to recommended dose of 21 mg/kg q12hr
- 6 months-4 years: 10 mg/kg PO q12hr, increase in increments of 10 mg/kg q12hr q2weeks to recommended dose of 25 mg/kg q12hr
- 4-16 years: 10 mg/kg PO q12hr; increase q2week by 10 mg/kg/dose to 30 mg/kg q12hr
- >16 years: 500 mg PO q12hr, increase by 500 mg q12hr q2weeks to recommended dose of 1500 mg q12hr
Immediate-release 3-D tablets (Spritam)
- Indicated for treatment of partial-onset seizures in patients aged >4 years
- <4 years: Safety and efficacy not established
- ≥4 years weighing 20-40 kg: 250 mg PO BID initially; increase daily dose q2wk by increments of 500 mg (250 mg BID) to a maximum daily dose of 1500 mg (750 mg BID)
- ≥4 years weighing >40 kg: 500 mg PO BID initially; increase daily dose q2wk by increments of 1000 mg (500 mg BID) to a maximum daily dose of 3000 mg (1500 mg BID)
Extended-release tablets (Keppra XR or Elepsia XR)
- Indicated as adjunctive therapy for treatment of partial-onset seizures in patients ≥12 years
- <12 years: Safety and efficacy not established
- &12 years: 1000 mg PO qDay initially; may adjust dose by 1000 mg increments q2wk to a maximum of 3000 mg/day
Primary Generalized Tonic-Clonic Seizures
<6 years: Safety and efficacy not established
Keppra
- 6-16 years: 10 mg/kg PO q12hr; increase q2week by 10 mg/kg/dose to recommended dose of 30 mg/kg q12hr; efficacy of doses <60 mg/kg/day not established
- >16 years: 500 mg PO q12hr, increase by 500 mg q12hr q2weeks to recommended dose of 1500 mg q12hr
Spritam
- ≥6 years weighing 20-40 kg: 250 mg PO BID initially; increase the daily dose q2wk by increments of 500 mg (250 mg BID) to a maximum recommended daily dose of 1500 mg/day (750 mg BID) ≥6 years weighing
- >40 kg: 500 mg PO BID initially; increase the daily dose q2wk by increments of 1000 mg (500 mg BID) to a maximum recommended daily dose of 3000 mg (1500 BID)
- Effectiveness of doses >3000 mg/day has not been adequately studied
Myoclonic Seizures
Keppra, Spritam
<12 years: Safety and efficacy not established
≥12 years: 500 mg PO q12hr; increase by 500 mg q12hr q2week to recommended dose of 1500 mg q12hr
Effectiveness of doses >3000 mg/day has not been studied
Neonatal Seizures (Orphan)
Orphan indication sponsor
- University of California; UCSD Medical Center 0935; La Jolla, CA 92093-0935
Dosing Considerations
Avoid abrupt withdrawal to reduce the risk of increased seizure frequency and status epilepticus
Interactions
Interaction Checker
No Results

Contraindicated
Serious - Use Alternative
Significant - Monitor Closely
Minor

Contraindicated (1)
- thalidomide
levetiracetam and thalidomide both increase sedation. Contraindicated.
Serious - Use Alternative (18)
- alfentanil
levetiracetam and alfentanil both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- buprenorphine
levetiracetam and buprenorphine both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- buprenorphine buccal
levetiracetam and buprenorphine buccal both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- codeine
levetiracetam and codeine both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- deferiprone
deferiprone, levetiracetam. Either increases toxicity of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Avoid use of deferiprone with other drugs known to be associated with neutropenia or agranulocytosis; if an alternative is not possible, monitor absolute neutrophil count more frequently.
- hydrocodone
levetiracetam and hydrocodone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- hydromorphone
levetiracetam and hydromorphone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- methadone
levetiracetam and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- methohexital
levetiracetam and methohexital both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- metoclopramide intranasal
levetiracetam, metoclopramide intranasal. Either increases effects of the other by Other (see comment). Avoid or Use Alternate Drug. Comment: Avoid use of metoclopramide intranasal or interacting drug, depending on importance of drug to patient.
- morphine
levetiracetam and morphine both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- oxycodone
levetiracetam and oxycodone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- oxymorphone
levetiracetam and oxymorphone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- pentobarbital
levetiracetam and pentobarbital both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- phenobarbital
levetiracetam and phenobarbital both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- ropeginterferon alfa 2b
ropeginterferon alfa 2b, levetiracetam. Either increases toxicity of the other by Other (see comment). Avoid or Use Alternate Drug. Comment: Myelosuppressive agents can produce additive myelosuppression. Avoid use and monitor patients receiving the combination for effects of excessive myelosuppression.
- tramadol
levetiracetam and tramadol both increase sedation. Avoid or Use Alternate Drug.
- triazolam
levetiracetam and triazolam both increase sedation. Avoid or Use Alternate Drug.
Monitor Closely (175)
- acalabrutinib
acalabrutinib, levetiracetam. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Coadministration may increase risk of myelosuppressive effects.
- acetaminophen/phenyltoloxamine
levetiracetam and acetaminophen/phenyltoloxamine both increase sedation. Use Caution/Monitor.
- acrivastine
acrivastine and levetiracetam both increase sedation. Use Caution/Monitor.
- alprazolam
levetiracetam and alprazolam both increase sedation. Use Caution/Monitor. Limit use to patients for whom alternative treatment options are inadequate
- amisulpride
amisulpride and levetiracetam both increase sedation. Use Caution/Monitor.
- amitriptyline
levetiracetam and amitriptyline both increase sedation. Use Caution/Monitor. Limit use to patients for whom alternative treatment options are inadequate
- amobarbital
levetiracetam and amobarbital both increase sedation. Use Caution/Monitor. Limit use to patients for whom alternative treatment options are inadequate
- amoxapine
levetiracetam and amoxapine both increase sedation. Use Caution/Monitor.
- aripiprazole
levetiracetam and aripiprazole both increase sedation. Use Caution/Monitor.
- asenapine
asenapine and levetiracetam both increase sedation. Use Caution/Monitor.
- asenapine transdermal
asenapine transdermal and levetiracetam both increase sedation. Use Caution/Monitor.
- avapritinib
avapritinib and levetiracetam both increase sedation. Use Caution/Monitor.
- baclofen
levetiracetam and baclofen both increase sedation. Use Caution/Monitor. Limit use to patients for whom alternative treatment options are inadequate
- benzhydrocodone/acetaminophen
benzhydrocodone/acetaminophen and levetiracetam both increase sedation. Use Caution/Monitor.
- betrixaban
levetiracetam will decrease the level or effect of betrixaban by unknown mechanism. Use Caution/Monitor.
- brexanolone
brexanolone, levetiracetam. Either increases toxicity of the other by sedation. Use Caution/Monitor.
- brexpiprazole
brexpiprazole and levetiracetam both increase sedation. Use Caution/Monitor.
- brimonidine
brimonidine and levetiracetam both increase sedation. Use Caution/Monitor.
- brivaracetam
brivaracetam, levetiracetam. Other (see comment). Use Caution/Monitor. Comment: Coadministration provided no added therapeutic benefit and may cause additive/synergistic adverse effects.
brivaracetam and levetiracetam both increase sedation. Use Caution/Monitor. - brompheniramine
levetiracetam and brompheniramine both increase sedation. Use Caution/Monitor.
- buprenorphine subdermal implant
buprenorphine subdermal implant and levetiracetam both increase sedation. Use Caution/Monitor.
- buprenorphine transdermal
buprenorphine transdermal and levetiracetam both increase sedation. Use Caution/Monitor.
- buprenorphine, long-acting injection
buprenorphine, long-acting injection and levetiracetam both increase sedation. Use Caution/Monitor.
- butabarbital
levetiracetam and butabarbital both increase sedation. Use Caution/Monitor.
- butalbital
levetiracetam and butalbital both increase sedation. Use Caution/Monitor.
- butorphanol
levetiracetam and butorphanol both increase sedation. Use Caution/Monitor.
- carbinoxamine
levetiracetam and carbinoxamine both increase sedation. Use Caution/Monitor.
- cariprazine
cariprazine and levetiracetam both increase sedation. Use Caution/Monitor.
- carisoprodol
levetiracetam and carisoprodol both increase sedation. Use Caution/Monitor.
- cenobamate
levetiracetam and cenobamate both increase sedation. Use Caution/Monitor.
- cetirizine
levetiracetam and cetirizine both increase sedation. Use Caution/Monitor.
- chlordiazepoxide
levetiracetam and chlordiazepoxide both increase sedation. Use Caution/Monitor.
- chlorpheniramine
levetiracetam and chlorpheniramine both increase sedation. Use Caution/Monitor.
- chlorpromazine
levetiracetam and chlorpromazine both increase sedation. Use Caution/Monitor.
- chlorzoxazone
levetiracetam and chlorzoxazone both increase sedation. Use Caution/Monitor.
- clemastine
levetiracetam and clemastine both increase sedation. Use Caution/Monitor.
- clobazam
levetiracetam and clobazam both increase sedation. Use Caution/Monitor.
- clomipramine
levetiracetam and clomipramine both increase sedation. Use Caution/Monitor.
- clonazepam
levetiracetam and clonazepam both increase sedation. Use Caution/Monitor.
- clonidine
levetiracetam and clonidine both increase sedation. Use Caution/Monitor.
- clorazepate
levetiracetam and clorazepate both increase sedation. Use Caution/Monitor.
- clozapine
levetiracetam and clozapine both increase sedation. Use Caution/Monitor.
- cyclobenzaprine
levetiracetam and cyclobenzaprine both increase sedation. Use Caution/Monitor.
- cyproheptadine
levetiracetam and cyproheptadine both increase sedation. Use Caution/Monitor.
- dabigatran
levetiracetam will decrease the level or effect of dabigatran by unknown mechanism. Use Caution/Monitor.
- dantrolene
levetiracetam and dantrolene both increase sedation. Use Caution/Monitor.
- daridorexant
levetiracetam and daridorexant both increase sedation. Use Caution/Monitor.
- desflurane
levetiracetam and desflurane both increase sedation. Use Caution/Monitor.
- desipramine
levetiracetam and desipramine both increase sedation. Use Caution/Monitor.
- deutetrabenazine
levetiracetam and deutetrabenazine both increase sedation. Use Caution/Monitor.
- dexbrompheniramine
levetiracetam and dexbrompheniramine both increase sedation. Use Caution/Monitor.
- dexchlorpheniramine
levetiracetam and dexchlorpheniramine both increase sedation. Use Caution/Monitor.
- diazepam
levetiracetam and diazepam both increase sedation. Use Caution/Monitor.
- difenoxin hcl
levetiracetam and difenoxin hcl both increase sedation. Use Caution/Monitor.
- dimenhydrinate
levetiracetam and dimenhydrinate both increase sedation. Use Caution/Monitor.
- diphenhydramine
levetiracetam and diphenhydramine both increase sedation. Use Caution/Monitor.
- diphenoxylate hcl
levetiracetam and diphenoxylate hcl both increase sedation. Use Caution/Monitor.
- doxepin
levetiracetam and doxepin both increase sedation. Use Caution/Monitor.
- doxylamine
levetiracetam and doxylamine both increase sedation. Use Caution/Monitor.
- droperidol
levetiracetam and droperidol both increase sedation. Use Caution/Monitor.
- edoxaban
levetiracetam will decrease the level or effect of edoxaban by unknown mechanism. Use Caution/Monitor.
- efavirenz
levetiracetam and efavirenz both increase sedation. Use Caution/Monitor.
- entacapone
levetiracetam and entacapone both increase sedation. Use Caution/Monitor.
- esketamine intranasal
esketamine intranasal, levetiracetam. Either increases toxicity of the other by sedation. Use Caution/Monitor.
- estazolam
levetiracetam and estazolam both increase sedation. Use Caution/Monitor.
- eszopiclone
levetiracetam and eszopiclone both increase sedation. Use Caution/Monitor.
- ethanol
levetiracetam and ethanol both increase sedation. Use Caution/Monitor.
- ethosuximide
levetiracetam and ethosuximide both increase sedation. Use Caution/Monitor.
- ethotoin
levetiracetam and ethotoin both increase sedation. Use Caution/Monitor.
- felbamate
levetiracetam and felbamate both increase sedation. Use Caution/Monitor.
- fenfluramine
levetiracetam and fenfluramine both increase sedation. Use Caution/Monitor.
- fentanyl
fentanyl and levetiracetam both increase sedation. Use Caution/Monitor.
- fentanyl intranasal
fentanyl intranasal and levetiracetam both increase sedation. Use Caution/Monitor.
- fentanyl iontophoretic transdermal system
fentanyl iontophoretic transdermal system and levetiracetam both increase sedation. Use Caution/Monitor.
- fentanyl transdermal
fentanyl transdermal and levetiracetam both increase sedation. Use Caution/Monitor.
- fentanyl transmucosal
fentanyl transmucosal and levetiracetam both increase sedation. Use Caution/Monitor.
- fexofenadine
levetiracetam and fexofenadine both increase sedation. Use Caution/Monitor.
- flibanserin
levetiracetam and flibanserin both increase sedation. Use Caution/Monitor.
- fluphenazine
levetiracetam and fluphenazine both increase sedation. Use Caution/Monitor.
- flurazepam
levetiracetam and flurazepam both increase sedation. Use Caution/Monitor.
- gabapentin
levetiracetam and gabapentin both increase sedation. Use Caution/Monitor.
- ganaxolone
levetiracetam and ganaxolone both increase sedation. Use Caution/Monitor.
- givinostat
levetiracetam and givinostat both increase QTc interval. Use Caution/Monitor. If coadministered, obtain ECGs when initiating, during concomitant use, and as clinically indicated. Withhold if QTc interval >500 ms or a change from baseline >60 ms.
- guanfacine
levetiracetam and guanfacine both increase sedation. Use Caution/Monitor.
- haloperidol
levetiracetam and haloperidol both increase sedation. Use Caution/Monitor.
- hydroxyzine
levetiracetam and hydroxyzine both increase sedation. Use Caution/Monitor.
- iloperidone
levetiracetam and iloperidone both increase sedation. Use Caution/Monitor.
- imipramine
levetiracetam and imipramine both increase sedation. Use Caution/Monitor.
- isoflurane
levetiracetam and isoflurane both increase sedation. Use Caution/Monitor.
- ketamine
levetiracetam and ketamine both increase sedation. Use Caution/Monitor.
- ketotifen, drug-eluting contact lens
levetiracetam and ketotifen, drug-eluting contact lens both increase sedation. Use Caution/Monitor.
- lamotrigine
levetiracetam and lamotrigine both increase sedation. Use Caution/Monitor.
- lasmiditan
lasmiditan, levetiracetam. Either increases effects of the other by sedation. Use Caution/Monitor. Coadministration of lasmiditan and other CNS depressant drugs, including alcohol have not been evaluated in clinical studies. Lasmiditan may cause sedation, as well as other cognitive and/or neuropsychiatric adverse reactions.
- lemborexant
lemborexant, levetiracetam. Either increases effects of the other by sedation. Modify Therapy/Monitor Closely. Dosage adjustment may be necessary if lemborexant is coadministered with other CNS depressants because of potentially additive effects.
- levocetirizine
levetiracetam and levocetirizine both increase sedation. Use Caution/Monitor.
- levorphanol
levetiracetam and levorphanol both increase sedation. Use Caution/Monitor.
- loratadine
levetiracetam and loratadine both increase sedation. Use Caution/Monitor.
- lorazepam
levetiracetam and lorazepam both increase sedation. Use Caution/Monitor.
- loxapine
levetiracetam and loxapine both increase sedation. Use Caution/Monitor.
- lumateperone
levetiracetam and lumateperone both increase sedation. Use Caution/Monitor.
- lurasidone
lurasidone, levetiracetam. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: Potential for increased CNS depressant effects when used concurrently; monitor for increased adverse effects and toxicity.
levetiracetam and lurasidone both increase sedation. Use Caution/Monitor. - maprotiline
levetiracetam and maprotiline both increase sedation. Use Caution/Monitor.
- meclizine
levetiracetam and meclizine both increase sedation. Use Caution/Monitor.
- meperidine
levetiracetam and meperidine both increase sedation. Use Caution/Monitor.
- meprobamate
levetiracetam and meprobamate both increase sedation. Use Caution/Monitor.
- metaxalone
levetiracetam and metaxalone both increase sedation. Use Caution/Monitor.
- methocarbamol
levetiracetam and methocarbamol both increase sedation. Use Caution/Monitor.
- methohexital
methohexital and levetiracetam both increase sedation. Use Caution/Monitor.
- methsuximide
levetiracetam and methsuximide both increase sedation. Use Caution/Monitor.
- midazolam
levetiracetam and midazolam both increase sedation. Use Caution/Monitor.
- midazolam intranasal
midazolam intranasal, levetiracetam. Either increases toxicity of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Concomitant use of barbiturates, alcohol, or other CNS depressants may increase the risk of hypoventilation, airway obstruction, desaturation, or apnea and may contribute to profound and/or prolonged drug effect.
- mirtazapine
levetiracetam and mirtazapine both increase sedation. Use Caution/Monitor.
- molindone
levetiracetam and molindone both increase sedation. Use Caution/Monitor.
- nalbuphine
levetiracetam and nalbuphine both increase sedation. Use Caution/Monitor.
- nitrous oxide
levetiracetam and nitrous oxide both increase sedation. Use Caution/Monitor.
- nortriptyline
levetiracetam and nortriptyline both increase sedation. Use Caution/Monitor.
- olanzapine
levetiracetam and olanzapine both increase sedation. Use Caution/Monitor.
- olanzapine/samidorphan
levetiracetam and olanzapine/samidorphan both increase sedation. Use Caution/Monitor.
- oliceridine
levetiracetam and oliceridine both increase sedation. Use Caution/Monitor.
- olopatadine intranasal
levetiracetam and olopatadine intranasal both increase sedation. Use Caution/Monitor.
- opicapone
levetiracetam and opicapone both increase sedation. Use Caution/Monitor.
- opium tincture
levetiracetam and opium tincture both increase sedation. Use Caution/Monitor.
- orlistat
orlistat decreases levels of levetiracetam by inhibition of GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Risk of convulsions.
- orphenadrine
levetiracetam and orphenadrine both increase sedation. Use Caution/Monitor.
- oxazepam
levetiracetam and oxazepam both increase sedation. Use Caution/Monitor.
- paliperidone
levetiracetam and paliperidone both increase sedation. Use Caution/Monitor.
- pentazocine
levetiracetam and pentazocine both increase sedation. Use Caution/Monitor.
- perampanel
levetiracetam and perampanel both increase sedation. Use Caution/Monitor.
- perphenazine
levetiracetam and perphenazine both increase sedation. Use Caution/Monitor.
- pheniramine
levetiracetam and pheniramine both increase sedation. Use Caution/Monitor.
- pimozide
levetiracetam and pimozide both increase sedation. Use Caution/Monitor.
- pomalidomide
levetiracetam and pomalidomide both increase sedation. Use Caution/Monitor.
- pregabalin
levetiracetam and pregabalin both increase sedation. Use Caution/Monitor.
- primidone
levetiracetam and primidone both increase sedation. Use Caution/Monitor.
- prochlorperazine
levetiracetam and prochlorperazine both increase sedation. Use Caution/Monitor.
- promethazine
levetiracetam and promethazine both increase sedation. Use Caution/Monitor.
- propofol
levetiracetam and propofol both increase sedation. Use Caution/Monitor.
- protriptyline
levetiracetam and protriptyline both increase sedation. Use Caution/Monitor.
- pyrilamine
levetiracetam and pyrilamine both increase sedation. Use Caution/Monitor.
- quazepam
levetiracetam and quazepam both increase sedation. Use Caution/Monitor.
- quetiapine
levetiracetam and quetiapine both increase sedation. Use Caution/Monitor.
- ramelteon
levetiracetam and ramelteon both increase sedation. Use Caution/Monitor.
- remifentanil
levetiracetam and remifentanil both increase sedation. Use Caution/Monitor.
- remimazolam
levetiracetam and remimazolam both increase sedation. Use Caution/Monitor.
- reserpine
levetiracetam and reserpine both increase sedation. Use Caution/Monitor.
- risperidone
levetiracetam and risperidone both increase sedation. Use Caution/Monitor.
- rivaroxaban
levetiracetam will decrease the level or effect of rivaroxaban by unknown mechanism. Use Caution/Monitor.
- scopolamine
levetiracetam and scopolamine both increase sedation. Use Caution/Monitor.
- sevelamer
sevelamer decreases levels of levetiracetam by increasing elimination. Use Caution/Monitor.
- sevoflurane
levetiracetam and sevoflurane both increase sedation. Use Caution/Monitor.
- sodium oxybate
levetiracetam and sodium oxybate both increase sedation. Use Caution/Monitor.
- stiripentol
stiripentol, levetiracetam. Either increases effects of the other by sedation. Use Caution/Monitor. Concomitant use stiripentol with other CNS depressants, including alcohol, may increase the risk of sedation and somnolence.
- sufentanil
levetiracetam and sufentanil both increase sedation. Use Caution/Monitor.
- sufentanil SL
levetiracetam and sufentanil SL both increase sedation. Use Caution/Monitor.
- suvorexant
levetiracetam and suvorexant both increase sedation. Use Caution/Monitor.
- tapentadol
levetiracetam and tapentadol both increase sedation. Use Caution/Monitor.
- tasimelteon
levetiracetam and tasimelteon both increase sedation. Use Caution/Monitor.
- temazepam
levetiracetam and temazepam both increase sedation. Use Caution/Monitor.
- tetrabenazine
levetiracetam and tetrabenazine both increase sedation. Use Caution/Monitor.
- thioridazine
levetiracetam and thioridazine both increase sedation. Use Caution/Monitor.
- thiothixene
levetiracetam and thiothixene both increase sedation. Use Caution/Monitor.
- tiagabine
levetiracetam and tiagabine both increase sedation. Use Caution/Monitor.
- tizanidine
levetiracetam and tizanidine both increase sedation. Use Caution/Monitor.
- tolcapone
levetiracetam and tolcapone both increase sedation. Use Caution/Monitor.
- topiramate
levetiracetam and topiramate both increase sedation. Use Caution/Monitor.
- trazodone
levetiracetam and trazodone both increase sedation. Use Caution/Monitor.
- trifluoperazine
levetiracetam and trifluoperazine both increase sedation. Use Caution/Monitor.
- triprolidine
levetiracetam and triprolidine both increase sedation. Use Caution/Monitor.
- valproic acid
levetiracetam and valproic acid both increase sedation. Use Caution/Monitor.
- vigabatrin
levetiracetam and vigabatrin both increase sedation. Use Caution/Monitor.
- zaleplon
levetiracetam and zaleplon both increase sedation. Use Caution/Monitor.
- ziconotide
levetiracetam and ziconotide both increase sedation. Use Caution/Monitor.
- ziprasidone
levetiracetam and ziprasidone both increase sedation. Use Caution/Monitor.
- zolpidem
levetiracetam and zolpidem both increase sedation. Use Caution/Monitor.
- zonisamide
levetiracetam and zonisamide both increase sedation. Use Caution/Monitor.
Minor (16)
- acetaminophen
levetiracetam decreases levels of acetaminophen by increasing metabolism. Minor/Significance Unknown. Enhanced metabolism incr levels of hepatotoxic metabolites.
- acetaminophen IV
levetiracetam decreases levels of acetaminophen IV by increasing metabolism. Minor/Significance Unknown. Enhanced metabolism incr levels of hepatotoxic metabolites.
- acetaminophen rectal
levetiracetam decreases levels of acetaminophen rectal by increasing metabolism. Minor/Significance Unknown. Enhanced metabolism incr levels of hepatotoxic metabolites.
- atracurium
levetiracetam decreases effects of atracurium by pharmacodynamic antagonism. Minor/Significance Unknown.
- biotin
levetiracetam decreases levels of biotin by unspecified interaction mechanism. Minor/Significance Unknown. Biotin supplementation may be necessary.
- cisatracurium
levetiracetam decreases effects of cisatracurium by pharmacodynamic antagonism. Minor/Significance Unknown.
- cyanocobalamin
levetiracetam decreases levels of cyanocobalamin by inhibition of GI absorption. Applies only to oral form of both agents. Minor/Significance Unknown.
- dexmethylphenidate
dexmethylphenidate increases effects of levetiracetam by decreasing metabolism. Minor/Significance Unknown.
- levocarnitine
levetiracetam decreases levels of levocarnitine by unspecified interaction mechanism. Minor/Significance Unknown.
- pancuronium
levetiracetam decreases effects of pancuronium by pharmacodynamic antagonism. Minor/Significance Unknown.
- rapacuronium
levetiracetam decreases effects of rapacuronium by pharmacodynamic antagonism. Minor/Significance Unknown.
- rocuronium
levetiracetam decreases effects of rocuronium by pharmacodynamic antagonism. Minor/Significance Unknown.
- sage
sage decreases effects of levetiracetam by pharmacodynamic antagonism. Minor/Significance Unknown. Theoretical interaction; some species of sage may cause convulsions.
- serdexmethylphenidate/dexmethylphenidate
serdexmethylphenidate/dexmethylphenidate increases effects of levetiracetam by decreasing metabolism. Minor/Significance Unknown.
- succinylcholine
levetiracetam decreases effects of succinylcholine by pharmacodynamic antagonism. Minor/Significance Unknown.
- vecuronium
levetiracetam decreases effects of vecuronium by pharmacodynamic antagonism. Minor/Significance Unknown.
Adverse Effects
>10%
Asthenia (11-15%)
Headache (14-19%)
Infection (11-15%)
Increased blood pressure (17% in children < 4 years)
Somnolence (11-15%)
Drowsiness (2-23%)
Fatigue (10-11%)
Anorexia (3-13%)
Weakness (9-15%)
Nasopharyngitis (7-15%)
Cough (2-11%)
1-10%
Viral infection (2%)
Asthma (2%)
Dizziness (5-9%)
Nervousness (2-10%)
Amnesia (2%)
Anxiety (2-3%)
Ataxia (3%)
Depression (2-5%)
Hostility (10%)
Paresthesia (2%)
Sinusitis (2%)
Diplopia (2%)
Amblyopia (2%)
Conjunctivitis (2-3%)
Albuminuria (4%)
<1%
Abnormal hepatic function tests
Dyskinesia
Eczema
Neutropenia
Decreased hematocrit
Leukopenia
Suicidal tendencies
Hepatitis
Pancreatitis
Bone marrow suppression
Epidermal necrolysis
Postmarketing Reports
Hepatic: Abnormal liver function tests, hepatic failure, hepatitis, pancreatitis
Skin: Alopecia, erythema multiforme; drug rash with eosinophilia and systemic syndrome (DRESS)
Neurologic: Choreoathetosis, dyskinesia, worsening of seizures
Hematology: Leukopenia, neutropenia, pancytopenia, thrombocytopenia, agranulocytosis
Skeletomuscular: Muscle weakness
Psychological: Panic attack
General: Weight loss
Hyponatremia
Acute kidney injury
Anaphylaxis and angioedema
Obsessive-compulsive disorders
Warnings
Contraindications
Hypersensitivity
Cautions
Somnolence and asthenia occurred most frequently within first 4 weeks of treatment; patients should be monitored for signs and symptoms and advised not to drive or operate machinery until they have gained sufficient experience on levetiracetam to gauge whether it adversely affects ability to drive or operate machinery
As with most antiepileptic drugs, drug should generally be withdrawn gradually because of risk of increased seizure frequency and status epilepticus; if withdrawal needed because of serious adverse reaction, rapid discontinuation can be considered
Psychiatric reactions: 13.3% of adults and 37.6% of children treated with levetiracetam reported nonpsychotic behavioral symptoms (eg, aggression, agitation, anger, anxiety, apathy, depersonalization, lability, hostility, hyperkinesis, irritability, nervousness, neurosis, and personality disorder) compared to 6.2% and 18.6% of adult and pediatric placebo patients respectively; dose reduction or discontinuation may be required
Monitor patients for behavioral abnormalities including psychotic symptoms, suicidal ideation, irritability, aggressive behavior, and for new or worsening depression, suicidal thoughts/behavior, and/or unusual changes in mood or behavior
Serious dermatological reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) reported; median time of onset is reported to be 14-17 days; if signs or symptoms suggest SJS/TEN, use of this drug should not be resumed and alternative therapy should be considered
May impair ability to operate heavy machinery
Therapy can cause hematologic abnormalities, including decreases in white blood cell (WBC), neutrophil, and red blood cell (RBC) counts; decreases in hemoglobin and hematocrit; and increases in eosinophil counts; cases of agranulocytosis, pancytopenia, and thrombocytopenia reported in postmarketing setting; a complete blood count is recommended in patients experiencing significant weakness, pyrexia, recurrent infections, or coagulation disorders
Increases in eosinophil counts observed
Monitor patients 1 month to <4 years of age for increases in diastolic blood pressure
Seizure control during pregnancy: Physiological changes during pregnancy may gradually decrease therapeutic plasma concentrations, this is particularly pronounced during the 3rd trimester; closely monitor serum levels during pregnancy and through the postpartum period
Agranulocytosis reported; in pediatric patients (4 to <16 years of age), statistically significant decreases in WBC and neutrophil counts were seen in patients treated with immediate-release levetiracetam; no patient was discontinued secondary to low WBC or neutrophil counts
One percent of adult patients and 2% of pediatric patients (4 to 16 years of age) treated with immediate-release levetiracetam experienced psychotic symptoms (paranoia)
Use caution in renal impairment; adjust dose; use immediate release formulation, instead of the extended release formulation, in patients with ESRD requiring hemodialysis
Can cause anaphylaxis or angioedema after the first dose or at any time during treatment; if patient develops signs or symptoms of anaphylaxis or angioedema, therapy should be discontinued and patient should seek immediate medical attention; therapy should be discontinued permanently if clear alternative etiology for reaction cannot be established
Drug reaction with eosinophilia and systemic symptoms (DRESS)
- DRESS, also known as multiorgan hypersensitivity, has been reported in patients taking antiepileptic drugs, including levetiracetam; these events can be fatal or life-threatening, particularly if diagnosis and treatment do not occur as early as possible
- DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling, in association with other organ system involvement, such as hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis, sometimes resembling an acute viral infection
- Eosinophilia is often present; because this disorder is variable in its expression, other organ systems not noted here may be involved; it is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident
- If such signs or symptoms are present, the patient should be evaluated immediately; this drug should be discontinued if an alternative etiology for signs or symptoms cannot be established
- Advise patients to avoid discontinuing medications without speaking to a healthcare provider
- Sudden discontinuation can lead to uncontrolled seizures
- Instruct patients to immediately go to emergency room if any unusual symptoms or reactions develop, including a rash, at any time while taking levetiracetam or clobazam
- Prompt recognition and early treatment is important for improving DRESS outcomes and decreasing mortality
- Symptoms of DRESS generally start 2-8 weeks after initiating
- DRESS can also be confused with other serious skin reactions (eg, SJS, TEN)
Pregnancy & Lactation
Pregnancy
There are no adequate and well-controlled studies in pregnant women; prolonged experience in pregnant women has not identified a drug-associated risk of major birth defects or miscarriage, based on published literature, which includes data from pregnancy registries, and reflects experience over two decades
In animal studies, levetiracetam produced evidence of developmental toxicity, including teratogenic effects, at doses similar to or greater than human therapeutic doses
Use during pregnancy only if the potential benefit justifies the potential risk to the fetus
Drug blood levels may decrease during pregnancy; physiological changes during pregnancy may affect drug concentration; decrease in drug plasma concentrations has been observed during pregnancy; this decrease is more pronounced during third trimester; dose adjustments may be necessary to maintain clinical response
Enroll patients in the North American Antiepileptic Drug pregnancy registry; 1-888-233-2334 or visit http://www.aedpregnancyregistry.org
Lactation
Drug is excreted in human milk; there are no data on effects of drug on breastfed infant, or on milk production
Unknown if distributed in human breast milk
Consider the developmental and health benefits of breastfeeding along with the mother’s clinical need for the drug, and any potential adverse effects on the breastfed infant from the drug or from the underlying maternal conditio
Pregnancy Categories
A: Generally acceptable. Controlled studies in pregnant women show no evidence of fetal risk.
B: May be acceptable. Either animal studies show no risk but human studies not available or animal studies showed minor risks and human studies done and showed no risk. C: Use with caution if benefits outweigh risks. Animal studies show risk and human studies not available or neither animal nor human studies done. D: Use in LIFE-THREATENING emergencies when no safer drug available. Positive evidence of human fetal risk. X: Do not use in pregnancy. Risks involved outweigh potential benefits. Safer alternatives exist. NA: Information not available.Pharmacology
Mechanism of Action
Antiepileptic mechanism unknown; may inhibit voltage-depedent N-type calcium channels; may bind to synaptic proteins that modulate neurotransmitter release; through displacement of negative modulators may facilitate GABA-ergic inhibitory transmission
Pyrrolidone derivative
Absorption
Bioavailability: 100%
Peak plasma time: 1 hr (immediate release); 4 hr (extended release)
Distribution
Protein bound: <10%
Metabolism
Metabolism: Hepatic enzymatic hydrolysis
Metabolites: Inactive
Elimination
Half-life: 6-8 hr (increased in elderly or renal disease)
Total body clearance: 0.96 mL/min/kg
Renal clearance: 0.6 mL/min/kg; 4 mL/min/kg (metabolite)
Clearance ~40% higher in children
Dialyzable: Yes
Excretion: Urine (66%)
Administration
IV Use
IV preparation: Dilute IV solution in 100 mL compatible solution
IV administration: Infuse IV over 15 minutes
Compatabilities
- Solutions: NS, LR, D5W
- Additive/syringe: lorazepam, diazepam, valproate sodium
Oral Administration
May take with or without food
Use oral solution for children who weigh ≤20 kg
Elepsia XR, Keppra XR: Swallow tablet whole; do not chew, split, crush, or cut tablets
Spritam
- Developed with ZipDose technology, which uses 3-dimensional printing to create a porous formulation of the antiepileptic that disintegrates rapidly with a sip of liquid, even at a high dose of up to 1000 mg
- May take with or without food
- Place the whole tablet on the tongue with a dry hand and follow with a sip of liquid
- Swallow only after tablet disintegrates in mouth (mean disintegration time 11 seconds [range 2-27 seconds])
- Tablet should not be swallowed intact
- Instruct patients not to push the tablet through the foil; the foil should be peeled from the blister by bending and lifting the peel tab around the blister seal so the table does not break
Storage
Unused vials: Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F)
May store diluted IV solution in PVC bags at 15-30°C (59-86°F) for up to 24 hr
Extended-release or immediate release tablets: Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F)
Solution: Store at 20-25°C (68-77°F)
Images
| BRAND | FORM. | UNIT PRICE | PILL IMAGE |
|---|---|---|---|
| Roweepra oral - | 1,000 mg tablet |  | |
| Roweepra oral - | 750 mg tablet |  | |
| Roweepra oral - | 500 mg tablet |  | |
| Elepsia XR oral - | 1,000 mg tablet |  | |
| levetiracetam oral - | 1,000 mg tablet |  | |
| levetiracetam oral - | 250 mg tablet |  | |
| levetiracetam oral - | 500 mg tablet |  | |
| levetiracetam oral - | 750 mg tablet |  | |
| levetiracetam oral - | 750 mg tablet |  | |
| levetiracetam oral - | 500 mg tablet |  | |
| levetiracetam oral - | 500 mg tablet |  | |
| levetiracetam oral - | 500 mg tablet |  | |
| levetiracetam oral - | 100 mg/mL solution |  | |
| levetiracetam oral - | 750 mg tablet |  | |
| levetiracetam oral - | 500 mg tablet |  | |
| levetiracetam oral - | 1,000 mg tablet |  | |
| levetiracetam oral - | 750 mg tablet |  | |
| levetiracetam oral - | 250 mg tablet |  | |
| levetiracetam oral - | 250 mg tablet | 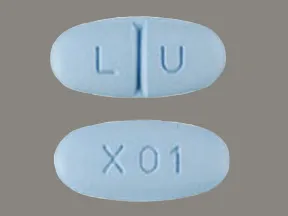 | |
| levetiracetam oral - | 100 mg/mL solution |  | |
| levetiracetam oral - | 1,000 mg tablet |  | |
| levetiracetam oral - | 500 mg tablet |  | |
| levetiracetam oral - | 750 mg tablet |  | |
| levetiracetam oral - | 500 mg tablet |  | |
| levetiracetam oral - | 250 mg tablet |  | |
| levetiracetam oral - | 750 mg tablet |  | |
| levetiracetam oral - | 250 mg tablet |  | |
| levetiracetam oral - | 750 mg tablet |  | |
| levetiracetam oral - | 500 mg tablet | 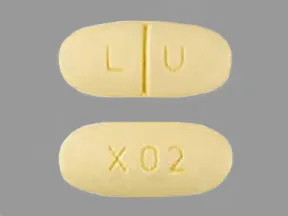 | |
| levetiracetam oral - | 250 mg tablet |  | |
| levetiracetam oral - | 1,000 mg tablet |  | |
| levetiracetam oral - | 1,000 mg tablet |  | |
| levetiracetam oral - | 750 mg tablet |  | |
| levetiracetam oral - | 750 mg tablet |  | |
| levetiracetam oral - | 750 mg tablet |  | |
| levetiracetam oral - | 100 mg/mL solution |  | |
| levetiracetam oral - | 500 mg tablet | 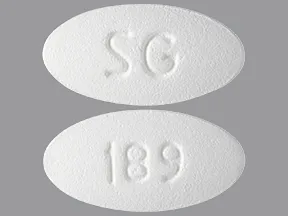 | |
| levetiracetam oral - | 100 mg/mL solution |  | |
| levetiracetam oral - | 500 mg tablet | 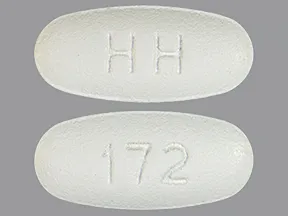 | |
| levetiracetam oral - | 500 mg tablet |  | |
| levetiracetam oral - | 1,000 mg tablet |  | |
| levetiracetam oral - | 100 mg/mL solution |  | |
| levetiracetam oral - | 250 mg tablet |  | |
| levetiracetam oral - | 250 mg tablet |  | |
| levetiracetam oral - | 100 mg/mL solution |  | |
| levetiracetam oral - | 1,000 mg tablet |  | |
| levetiracetam oral - | 500 mg tablet |  | |
| levetiracetam oral - | 1,000 mg tablet |  | |
| levetiracetam oral - | 500 mg tablet |  | |
| levetiracetam oral - | 750 mg tablet |  | |
| levetiracetam oral - | 100 mg/mL solution |  | |
| levetiracetam oral - | 500 mg tablet |  | |
| levetiracetam oral - | 250 mg tablet |  | |
| levetiracetam oral - | 500 mg tablet |  | |
| levetiracetam oral - | 750 mg tablet |  | |
| levetiracetam oral - | 100 mg/mL solution |  | |
| levetiracetam oral - | 750 mg tablet |  | |
| levetiracetam oral - | 750 mg tablet |  | |
| levetiracetam oral - | 500 mg tablet |  | |
| levetiracetam oral - | 250 mg tablet |  | |
| levetiracetam oral - | 100 mg/mL solution |  | |
| levetiracetam intravenous - | 500 mg/5 mL vial |  | |
| levetiracetam intravenous - | 500 mg/5 mL vial |  | |
| levetiracetam intravenous - | 500 mg/5 mL vial |  | |
| levetiracetam intravenous - | 500 mg/5 mL vial |  | |
| levetiracetam intravenous - | 500 mg/5 mL vial |  | |
| levetiracetam intravenous - | 500 mg/5 mL vial |  | |
| levetiracetam intravenous - | 500 mg/5 mL vial |  | |
| levetiracetam intravenous - | 500 mg/5 mL vial |  | |
| levetiracetam intravenous - | 500 mg/5 mL vial |  | |
| levetiracetam intravenous - | 500 mg/5 mL vial |  | |
| levetiracetam intravenous - | 500 mg/5 mL vial |  | |
| levetiracetam intravenous - | 500 mg/5 mL vial |  | |
| levetiracetam intravenous - | 500 mg/5 mL vial |  | |
| Keppra intravenous - | 500 mg/5 mL vial |  | |
| Spritam oral - | 1,000 mg tablet |  | |
| Spritam oral - | 750 mg tablet |  | |
| Spritam oral - | 500 mg tablet |  | |
| Spritam oral - | 250 mg tablet |  | |
| Roweepra XR oral - | 750 mg tablet |  | |
| Roweepra XR oral - | 500 mg tablet |  | |
| Keppra oral - | 1,000 mg tablet |  | |
| Keppra oral - | 250 mg tablet |  | |
| Keppra oral - | 750 mg tablet |  | |
| Keppra oral - | 500 mg tablet |  | |
| Keppra oral - | 100 mg/mL solution |  | |
| Keppra XR oral - | 500 mg tablet |  | |
| Keppra XR oral - | 750 mg tablet |  |
Copyright © 2010 First DataBank, Inc.
Patient Handout
levetiracetam oral
LEVETIRACETAM TABLET - FOR ORAL SUSPENSION
(LEE-ve-tye-RA-se-tam)
COMMON BRAND NAME(S): Spritam
USES: Levetiracetam is used with other medications to treat seizures (epilepsy). It belongs to a class of drugs known as anticonvulsants. Levetiracetam may decrease the number of seizures you have.
HOW TO USE: Read the Medication Guide provided by your pharmacist before you start taking levetiracetam and each time you get a refill. If you have any questions, ask your doctor or pharmacist.Take this medication by mouth with or without food as directed by your doctor, usually twice a day.Carefully remove the tablet(s) from the foil packet as directed by the product package. Do not push the tablet(s) through the foil. Dry your hands before handling the medication. Place each dose on the tongue and take a sip of liquid. Allow the medication to completely dissolve before swallowing it. Do not swallow the tablet(s) whole.The tablet(s) may also be placed in a cup with a small amount of liquid (1 tablespoon/15 milliliters). Swirl the mixture gently, then drink all of the mixture right away. To make sure you have taken all of the medication, add another small amount of liquid to the cup to rinse it, and drink it right away.The dosage is based on your medical condition and response to treatment. The dosage in children is also based on weight. To reduce your risk of side effects (such as dizziness and drowsiness), your doctor may direct you to start this medication at a low dose and gradually increase your dose. Follow your doctor's instructions carefully.Use this medication regularly to get the most benefit from it. To help you remember, take it at the same times each day.Do not increase your dose or use this drug more often or for longer than prescribed. Your condition will not improve any faster, and your risk of side effects will increase.Do not stop taking this medication without consulting your doctor. Your seizures may become worse when the drug is suddenly stopped. Your dose should be gradually decreased.Tell your doctor if your seizures lasts, change, or gets worse.
SIDE EFFECTS: Drowsiness, dizziness, unusual tiredness, or weakness may occur. These side effects are more common during the first 4 weeks and usually lessen as your body adjusts to the medication. If any of these effects last or get worse, tell your doctor or pharmacist promptly.Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.Tell your doctor right away if you have any serious side effects, such as: loss of coordination (such as difficulty walking and controlling muscles), mental/mood changes (such as irritability, aggression, agitation, anger, anxiety), signs of anemia (such as unusual tiredness that doesn't go away, pale skin, fast breathing, fast heartbeat), easy bruising/bleeding.A small number of people who take anticonvulsants for any condition (such as seizures, bipolar disorder, pain) may experience depression, suicidal thoughts/attempts, or other mental/mood problems. Tell your doctor right away if you or your family/caregiver notice any unusual/sudden changes in your mood, thoughts, or behavior including signs of depression, suicidal thoughts/attempts, thoughts about harming yourself.Levetiracetam can commonly cause a rash that is usually not serious. However, you may not be able to tell it apart from a rare rash that could be a sign of a severe reaction. Tell your doctor right away if you develop any rash.A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, such as: fever, swollen lymph nodes, rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.In the US -Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
PRECAUTIONS: Before taking levetiracetam, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.Before using this medication, tell your doctor or pharmacist your medical history, especially of: kidney disease (such as dialysis treatment), mental/mood disorders (such as depression).This drug may make you dizzy or drowsy, especially during the first month of treatment. Alcohol or marijuana (cannabis) can make you more dizzy or drowsy. Do not drive, use machinery, ride a bicycle, or do anything that needs alertness until you can do it safely. Limit alcoholic beverages. Talk to your doctor if you are using marijuana (cannabis).Before having surgery, tell your doctor or dentist about all the products you use (such as prescription drugs, nonprescription drugs, and herbal products).Children may be more sensitive to the side effects of the drug, especially mental/mood changes (such as irritability, aggression, agitation, anger, anxiety, depression, thoughts of suicide). Children younger than 4 years may be at greater risk for increased blood pressure while using this drug (see also Notes section).Older adults may be more sensitive to the side effects of this drug, especially drowsiness, dizziness or loss of coordination. These side effects can increase the risk of falling.During pregnancy, this medication should be used only when clearly needed. Discuss the risks and benefits with your doctor.This medication passes into breast milk. Consult your doctor before breastfeeding.
DRUG INTERACTIONS: Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.A product that may interact with this drug is: orlistat.
OVERDOSE: If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center. Symptoms of overdose may include: slow/shallow breathing, loss of consciousness.
NOTES: Do not share this medication with others.Lab and/or medical tests (such as kidney function, complete blood count) may be done while you are taking this medication. In children younger than 4 years, blood pressure may also be monitored. Consult your doctor for more details.
MISSED DOSE: If you miss a dose, take it as soon as you remember. If it is near the time of the next dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
STORAGE: Store at room temperature away from light and moisture. Do not store in the bathroom. Keep all medications away from children and pets.Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
MEDICAL ALERT: Your condition can cause complications in a medical emergency. For information about enrolling in MedicAlert, call 1-888-633-4298 (US) or 1-800-668-1507 (Canada).
Information last revised March 2024. Copyright(c) 2024 First Databank, Inc.
IMPORTANT: HOW TO USE THIS INFORMATION: This is a summary and does NOT have all possible information about this product. This information does not assure that this product is safe, effective, or appropriate for you. This information is not individual medical advice and does not substitute for the advice of your health care professional. Always ask your health care professional for complete information about this product and your specific health needs.
Formulary
Adding plans allows you to compare formulary status to other drugs in the same class.
To view formulary information first create a list of plans. Your list will be saved and can be edited at any time.
Adding plans allows you to:
- View the formulary and any restrictions for each plan.
- Manage and view all your plans together – even plans in different states.
- Compare formulary status to other drugs in the same class.
- Access your plan list on any device – mobile or desktop.