Dosing & Uses
Hyperlipidemias
Initial: 10-20 mg PO qDay
Patients who require reduction in LDL-C >45%: May start at 40 mg qDay
Dosage range: 10-80 mg qDay
Indications
- Adjunct to other LDL-C-lowering therapies, or alone if such treatments are unavailable, to reduce LDL-C homozygous familial hypercholesterolemia (HoFH)
- As an adjunct to diet for treatment of primary dysbetalipoproteinemia or hypertriglyceridemia
-
Adjunct to diet to reduce LDL-C in
- Primary hyperlipidemia
- Heterozygous familial hypercholesterolemia (HeFH)
Cardiovascular Disease Prevention
Initial: 10-20 mg PO qDay
Patients who require reduction in LDL-C >45%: May start at 40 mg qDay
Dosage range: 10-80 mg qDay
Indicated to reduce risk of
- Myocardial infarction (MI), stroke, revascularization procedures, and angina in adults with multiple risk factors for coronary heart disease (CHD), but without clinically evident CHD
- MI and stroke in adults with type 2 diabetes mellitus with multiple risk factors for CHD, but without clinically evident CHD
- Non-fatal MI, Fatal and non-fatal stroke, revascularization procedures, hospitalization for CHF, and angina in adults with clinically evident CHD
Dosage Modifications
Renal impairment
- Renal impairment does not affect atorvastatin plasma concentrations, therefore, there is no dosage adjustment
- Renal impairment is a risk factor for myopathy and rhabdomyolysis
- Monitor all patients with renal impairment for development of myopathy
Hepatic impairment
- In patients with chronic alcoholic liver disease, plasma concentrations are markedly increased
- Mild (Child-Pugh A): Cmax and AUC are each 4-fold greater
- Moderate (Child-Pugh B): Cmax and AUC are ~16-fold and 11-fold increased, respectively
- Acute liver failure or decompensated cirrhosis: Contraindicated
Coadministration with antivirals
- Saquinavir plus ritonavir, darunavir plus ritonavir, fosamprenavir, fosamprenavir plus ritonavir, elbasvir/grazoprevir, or letermovir: Not to exceed 20 mg qDay
- Nelfinavir: Not to exceed 40 mg qDay
Coadministration with select azole antifungals or macrolide antibiotics
- Clarithromycin or itraconazole: Not to exceed 20 mg qDay
Dosing Considerations
Assess LDL-C when clinically appropriate, as early as 4 weeks after initiating, and adjust dosage if necessary
Dosage Forms & Strengths
tablet (Lipitor, generic)
- 10mg
- 20mg
- 40mg
- 80mg
oral suspension (Atorvaliq)
- 20mg/5mL
Heterozygous Familial Hypercholesterolemia
Indicated as an adjunct to diet to reduce LDL-C in in patients aged >10 years with heterozygous familial hypercholesterolemia (HeFH)
<10 years: Safety and efficacy not established
≥10 years: 10 mg PO qDay initially; titrate at 4-week intervals; not to exceed 20 mg PO qDay
Homozygous Familial Hypercholesterolemia
Indicated as an adjunct to other LCL-C lowering therapies, or alone if such treatment are unavailable, to reduce LCL-C in patients aged >10 years with homozygous familial hypercholesterolemia (HoFH)
<10 years: Safety and efficacy not established
≥10 years: 10-20 mg PO qDay initially
Dosage range: 10-80 mg qDay
Dosage Modifications
Renal impairment
- Renal impairment does not affect atorvastatin plasma concentrations, therefore, there is no dosage adjustment
- Renal impairment is a risk factor for myopathy and rhabdomyolysis
- Monitor all patients with renal impairment for development of myopathy
Hepatic impairment
- In patients with chronic alcoholic liver disease, plasma concentrations are markedly increased
- Mild (Child-Pugh A): Cmax and AUC are each 4-fold greater
- Moderate (Child-Pugh B): Cmax and AUC are ~16-fold and 11-fold increased, respectively
- Acute liver failure or decompensated cirrhosis: Contraindicated
Coadministration with antivirals
- Saquinavir plus ritonavir, darunavir plus ritonavir, fosamprenavir, fosamprenavir plus ritonavir, elbasvir/grazoprevir, or letermovir: Not to exceed 20 mg qDay
- Nelfinavir: Not to exceed 40 mg qDay
Coadministration with select azole antifungals or macrolide antibiotics
- Clarithromycin or itraconazole: Not to exceed 20 mg qDay
Dosing Considerations
Assess LDL-C when clinically appropriate, as early as 4 weeks after initiating, and adjust dosage if necessary
Interactions
Interaction Checker
No Results
Contraindicated
Serious - Use Alternative
Significant - Monitor Closely
Minor
Contraindicated (6)
- cyclosporine
cyclosporine increases toxicity of atorvastatin by Other (see comment). Contraindicated. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- gemfibrozil
gemfibrozil increases toxicity of atorvastatin by Other (see comment). Contraindicated. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- lonafarnib
lonafarnib will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Contraindicated.
- pazopanib
atorvastatin increases toxicity of pazopanib by P-glycoprotein (MDR1) efflux transporter. Contraindicated.
- red yeast rice
atorvastatin, red yeast rice. Either increases toxicity of the other by pharmacodynamic synergism. Contraindicated. May increase creatine kinase levels and increase risk of myopathy or rhabdomyolysis; red yeast rice contains monocolin K (reportedly identical to lovastatin).
- tipranavir
tipranavir increases levels of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Contraindicated. This interaction is the net effect of tipranavir being coadministered with ritonavir (boosted therapy); increased risk of myopathy including rhabdomyolysis.
Serious - Use Alternative (60)
- afatinib
atorvastatin increases levels of afatinib by P-glycoprotein (MDR1) efflux transporter. Avoid or Use Alternate Drug. Reduce afatinib daily dose by 10 mg if not tolerated when coadministered with P-gp inhibitors.
- apalutamide
apalutamide will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Coadministration of apalutamide, a strong CYP3A4 inducer, with drugs that are CYP3A4 substrates can result in lower exposure to these medications. Avoid or substitute another drug for these medications when possible. Evaluate for loss of therapeutic effect if medication must be coadministered. Adjust dose according to prescribing information if needed.
- bosutinib
atorvastatin increases levels of bosutinib by P-glycoprotein (MDR1) efflux transporter. Avoid or Use Alternate Drug.
- carbamazepine
carbamazepine will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- ceftobiprole medocaril sodium
ceftobiprole medocaril sodium will increase the level or effect of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Ceftobiprole (an OATP1B1/1B3 inhibitor) may increase plasma concentrations of OATP1B1 and OATP1B3 substrates.
- ceritinib
ceritinib will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- cimetidine
cimetidine will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- clarithromycin
clarithromycin will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Do not exceed atorvastatin dose of 20 mg/day when coadministered with clarithromycin
clarithromycin will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Avoid or Use Alternate Drug. Do not exceed atorvastatin dose of 20 mg/day when coadministered with clarithromycin
clarithromycin increases toxicity of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: OATP1B1 inhibitors may increase risk of myopathy. - colchicine
colchicine, atorvastatin. Either increases toxicity of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Increased risk of rhabdomyolysis (incl a fatality).
- cyclosporine
cyclosporine will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Coadministration increases risk of statin-associated myopathy including rhabdomyolysis
- darolutamide
darolutamide will increase the level or effect of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Darolutamide is a BCRP inhibitor. Avoid coadministration with BCRP inhibitors. If use is unavoidable, closely monitor for adverse reactions and consider dose reduction of BCRP substrate drug (refer BCRP substrate prescribing information).
- edoxaban
atorvastatin will increase the level or effect of edoxaban by P-glycoprotein (MDR1) efflux transporter. Avoid or Use Alternate Drug. Dose adjustment may be required with strong P-gp inhibitors. DVT/PE treatment: Decrease dose to 30 mg PO once daily. NVAF: No dose reduction recommended
- eltrombopag
eltrombopag increases toxicity of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- eluxadoline
atorvastatin increases levels of eluxadoline by decreasing metabolism. Avoid or Use Alternate Drug. Decrease eluxadoline dose to 75 mg PO BID if coadministered with OATP1B1 inhibitors. .
- enzalutamide
enzalutamide will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- erdafitinib
erdafitinib will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Avoid or Use Alternate Drug. If coadministration unavoidable, separate administration by at least 6 hr before or after administration of P-gp substrates with narrow therapeutic index.
- erythromycin base
erythromycin base will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
erythromycin base increases toxicity of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: OATP1B1 inhibitors may increase risk of myopathy. - erythromycin ethylsuccinate
erythromycin ethylsuccinate will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
erythromycin ethylsuccinate increases toxicity of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: OATP1B1 inhibitors may increase risk of myopathy. - erythromycin lactobionate
erythromycin lactobionate will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
erythromycin lactobionate increases toxicity of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: OATP1B1 inhibitors may increase risk of myopathy. - erythromycin stearate
erythromycin stearate will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
erythromycin stearate increases toxicity of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: OATP1B1 inhibitors may increase risk of myopathy. - everolimus
atorvastatin will increase the level or effect of everolimus by P-glycoprotein (MDR1) efflux transporter. Contraindicated.
- fenofibrate
fenofibrate, atorvastatin. Either increases effects of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Fenofibrate may further increase risk for rhabdomyolysis when added to optimal statin regimen to further decrease TG and increase HDLs.
- fenofibrate micronized
fenofibrate micronized, atorvastatin. Either increases effects of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Fenofibrate may further increase risk for rhabdomyolysis when added to optimal statin regimen to further decrease TG and increase HDLs.
- fenofibric acid
fenofibric acid, atorvastatin. Either increases effects of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Fenofibrate may further increase risk for rhabdomyolysis when added to optimal statin regimen to further decrease TG and increase HDLs.
- fexinidazole
fexinidazole will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Fexinidazole inhibits CYP3A4. Coadministration may increase risk for adverse effects of CYP3A4 substrates.
- fosamprenavir
fosamprenavir will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Limit atorvastatin dose to 20 mg/day
- gemfibrozil
gemfibrozil, atorvastatin. Either increases effects of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Gemfibrozil may further increase risk for rhabdomyolysis when added to optimal statin regimen to further decrease TG and increase HDLs.
- glecaprevir/pibrentasvir
glecaprevir/pibrentasvir increases levels of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: Increased statin concentrations resulting from OATP1B1 inhibition may increase risk of myopathy, including rhabdomyolysis. Coadministration of glecaprevir/pibrentasvir with atorvastatin is not recommended.
- idelalisib
idelalisib will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Idelalisib is a strong CYP3A inhibitor; avoid coadministration with sensitive CYP3A substrates
- indinavir
indinavir increases toxicity of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Risk of myopathy and rhabdomyolysis increased when atorvastatin coadministered with CYP3A4 inhibitors; use lowest statin dose possible.
indinavir increases toxicity of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: OATP1B1 inhibitors may increase risk of myopathy. - itraconazole
itraconazole will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Limit atorvastatin dose to 20 mg/day
- ivosidenib
ivosidenib will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Avoid coadministration of sensitive CYP3A4 substrates with ivosidenib or replace with alternate therapies. If coadministration is unavoidable, monitor patients for loss of therapeutic effect of these drugs.
- lasmiditan
lasmiditan increases levels of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Avoid or Use Alternate Drug.
lasmiditan increases levels of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: Lasmiditan inhibits BCRP in vitro. Avoid coadministration of lasmiditan with BCRP substrates. - leniolisib
leniolisib will increase the level or effect of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Leniolisib, a BCRP, OATP1B1, and OATP1B3 inhibitor, may increase systemic exposure of these substrates
- levoketoconazole
levoketoconazole increases toxicity of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- lopinavir
lopinavir increases levels of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. If coadministration cannot be avoided, limit atorvastatin dose to 20 mg/day and monitor for signs and symptoms of toxicity, including liver function tests abnormalities, myalgia and rhabdomyolysis.
- mesterolone
mesterolone increases toxicity of atorvastatin by decreasing metabolism. Avoid or Use Alternate Drug. Risk of rhabdomyolysis (theoretical interaction based on case reports of combination of danazol and >20 mg/day lovastatin).
- mifepristone
mifepristone increases toxicity of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- nefazodone
nefazodone will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- nelfinavir
nelfinavir increases toxicity of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- niacin
niacin, atorvastatin. Either increases toxicity of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Increased risk of rhabdomyolysis (>1 g/day niacin).
- ombitasvir/paritaprevir/ritonavir & dasabuvir (DSC)
ombitasvir/paritaprevir/ritonavir & dasabuvir (DSC) increases toxicity of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- pomalidomide
atorvastatin increases levels of pomalidomide by P-glycoprotein (MDR1) efflux transporter. Avoid or Use Alternate Drug.
- pretomanid
atorvastatin, pretomanid. Either increases toxicity of the other by Other (see comment). Avoid or Use Alternate Drug. Comment: Pretomanid regimen associated with hepatotoxicity. Avoid alcohol and hepatotoxic agents, including herbal supplements and drugs other than bedaquiline and linezolid.
- quinidine
quinidine will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Avoid or Use Alternate Drug.
- repotrectinib
atorvastatin will increase the level or effect of repotrectinib by P-glycoprotein (MDR1) efflux transporter. Avoid or Use Alternate Drug.
- rifabutin
rifabutin will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- rifampin
rifampin will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
rifampin increases toxicity of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: OATP1B1 inhibitors may increase risk of myopathy. - rimegepant
atorvastatin will increase the level or effect of rimegepant by P-glycoprotein (MDR1) efflux transporter. Avoid or Use Alternate Drug.
- riociguat
atorvastatin will increase the level or effect of riociguat by decreasing metabolism. Avoid or Use Alternate Drug. Coadministration of riociguat (substrate of CYP isoenzymes 1A1, 2C8, 3A, 2J2) with strong CYP inhibitors may require a decreased initial dose of 0.5 mg PO TID; monitor for signs of hypotension and reduce dose if needed
- ritonavir
ritonavir increases toxicity of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- saquinavir
saquinavir will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Limit atorvastatin dose to 20 mg/day
saquinavir increases toxicity of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Comment: OATP1B1 inhibitors may increase risk of myopathy. - sotorasib
sotorasib will decrease the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Avoid or Use Alternate Drug. If use is unavoidable, refer to the prescribing information of the P-gp substrate for dosage modifications.
- St John's Wort
St John's Wort will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- tepotinib
tepotinib will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Avoid or Use Alternate Drug. If concomitant use unavoidable, reduce the P-gp substrate dosage if recommended in its approved product labeling.
- topotecan
atorvastatin will increase the level or effect of topotecan by P-glycoprotein (MDR1) efflux transporter. Avoid or Use Alternate Drug. Product labeling for PO topotecan recommends avoiding concomitant use of P-gp inhibitors; the interaction with IV topotecan may be less severe but is still likely of clinical significance
- trofinetide
trofinetide will increase the level or effect of atorvastatin by Other (see comment). Avoid or Use Alternate Drug. Trofinetide (an OATP131 and OATP13B inhibitor) may increase plasma levels of OATP131 or OATP13B substrates. Avoid coadministration with sensitive substrates.
- tucatinib
tucatinib will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Avoid concomitant use of tucatinib with CYP3A substrates, where minimal concentration changes may lead to serious or life-threatening toxicities. If unavoidable, reduce CYP3A substrate dose according to product labeling.
- venetoclax
atorvastatin will increase the level or effect of venetoclax by P-glycoprotein (MDR1) efflux transporter. Avoid or Use Alternate Drug. If a P-gp inhibitor must be used, reduce the venetoclax dose by at least 50%. Monitor more closely for signs of venetoclax toxicities.
- voxelotor
voxelotor will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Voxelotor increases systemic exposure of sensitive CYP3A4 substrates. Avoid coadministration with sensitive CYP3A4 substrates with a narrow therapeutic index. Consider dose reduction of the sensitive CYP3A4 substrate(s) if unable to avoid.
Monitor Closely (200)
- acalabrutinib
acalabrutinib increases levels of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: Acalabrutinib may increase exposure to coadministered BCRP substrates by inhibition of intestinal BCRP.
- albiglutide
albiglutide will decrease the level or effect of atorvastatin by Other (see comment). Use Caution/Monitor. Based on pharmacokinetic studies, atorvastatin Cmax decreased by 38% and median Tmax delayed from 1h to 3h and the AUC did not change.
- aliskiren
atorvastatin will increase the level or effect of aliskiren by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
atorvastatin increases levels of aliskiren by unspecified interaction mechanism. Use Caution/Monitor. - amiodarone
amiodarone will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- amitriptyline
atorvastatin will increase the level or effect of amitriptyline by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- apalutamide
apalutamide will decrease the level or effect of atorvastatin by increasing elimination. Use Caution/Monitor. Apalutamide induces UGT and weakly induces BCRP and OATP1B1. Drugs that are eliminated via these pathways may have decreased systemic exposure if coadministered with apalutamide.
- aprepitant
aprepitant will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- armodafinil
armodafinil will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- artemether/lumefantrine
artemether/lumefantrine will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- asciminib
asciminib will increase the level or effect of atorvastatin by Other (see comment). Modify Therapy/Monitor Closely. Asciminib is an OATP1B and BCRP inhibitor; closely monitor for adverse reactions in patients treated at all recommended doses with concomitant use of other OATP1B or BCRP substrates; reduce dosage of other OATP1B or BCRP substrates as recommended in their Prescribing Information when used concomitantly at all recommended doses
- atazanavir
atazanavir will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- atogepant
atorvastatin will increase the level or effect of atogepant by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- avapritinib
atorvastatin will increase the level or effect of avapritinib by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- axitinib
atorvastatin increases levels of axitinib by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- azithromycin
azithromycin will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. If this combination is used, closely monitor for evidence of atorvastatin toxicity (eg, muscle aches or pains, renal dysfunction).
- bazedoxifene/conjugated estrogens
atorvastatin will increase the level or effect of bazedoxifene/conjugated estrogens by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- belzutifan
belzutifan will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. If unable to avoid coadministration of belzutifan with sensitive CYP3A4 substrates, consider increasing the sensitive CYP3A4 substrate dose in accordance with its prescribing information.
- berotralstat
atorvastatin increases levels of berotralstat by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
berotralstat will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. Monitor or titrate P-gp substrate dose if coadministered. - betrixaban
atorvastatin increases levels of betrixaban by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. Decrease betrixaban dose to 80 mg PO once, then 40 mg PO qDay if coadministered with a P-gp inhibitor.
- bosentan
bosentan will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- bosutinib
bosutinib increases levels of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- budesonide
atorvastatin will increase the level or effect of budesonide by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- butabarbital
butabarbital will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- butalbital
butalbital will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- carbamazepine
carbamazepine increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- caspofungin
caspofungin increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- cenobamate
cenobamate will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Increase dose of CYP3A4 substrate, as needed, when coadministered with cenobamate.
- ceritinib
atorvastatin increases levels of ceritinib by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- cholestyramine
cholestyramine decreases levels of atorvastatin by inhibition of GI absorption. Applies only to oral form of both agents. Use Caution/Monitor.
- cholic acid
atorvastatin increases toxicity of cholic acid by decreasing elimination. Modify Therapy/Monitor Closely. Avoid concomitant use of inhibitors of the bile salt efflux pump (BSEP). May exacerbate accumulation of conjugated bile salts in the liver and result in clinical symptoms. If concomitant use is necessary, monitor serum transaminases and bilirubin.
- clotrimazole
clotrimazole will decrease the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
clotrimazole increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy. - cobicistat
cobicistat will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. For HMG-CoA reductase inhibitors that are not contraindicated with cobicistat, dose should not exceed 20 mg/day.
- conivaptan
conivaptan will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- conjugated estrogens
atorvastatin will increase the level or effect of conjugated estrogens by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- conjugated estrogens, vaginal
atorvastatin will increase the level or effect of conjugated estrogens, vaginal by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- cortisone
atorvastatin will increase the level or effect of cortisone by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- crizotinib
crizotinib increases levels of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Dose reduction may be needed for coadministered drugs that are predominantly metabolized by CYP3A.
crizotinib increases levels of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - crofelemer
crofelemer increases levels of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Crofelemer has the potential to inhibit CYP3A4 at concentrations expected in the gut; unlikely to inhibit systemically because minimally absorbed.
- dabigatran
atorvastatin will increase the level or effect of dabigatran by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. Atrial fibrillation: Avoid coadministering dabigatran with P-gp inhibitors if CrCl <30 mL/min. DVT/PE treatment: Avoid coadministering dabigatran with P-gp inhibitors if CrCl <50 mL/min
- dabrafenib
dabrafenib will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely.
- danazol
danazol will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- danicopan
danicopan will increase the level or effect of atorvastatin by Other (see comment). Use Caution/Monitor. Danicopan increases plasma concentrations of BCRP substrates; consider dose reduction of BCRP substrate according to its prescribing information.
danicopan will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. Danicopan increases plasma concentrations of P-gp substrates; consider dose reduction of P-gp substrates where minimal concentration changes may lead to serious adverse reactions. - daptomycin
atorvastatin, daptomycin. Either increases toxicity of the other by Other (see comment). Modify Therapy/Monitor Closely. Comment: Coadministration of daptomycin with HMG-CoA reductase inhibitors may increase CPK levels and risk for myopathy; consider temporary suspension of HMG-CoA reductase inhibitors during daptomycin therapy.
- darifenacin
darifenacin will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- darunavir
darunavir will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. For HMG-CoA reductase inhibitors that are not contraindicated with darunavir, dose should not exceed 20 mg/day.
- dasatinib
dasatinib will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- daunorubicin
atorvastatin will increase the level or effect of daunorubicin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- deferasirox
deferasirox will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- deflazacort
atorvastatin will increase the level or effect of deflazacort by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- dexamethasone
dexamethasone will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
atorvastatin will increase the level or effect of dexamethasone by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - DHEA, herbal
DHEA, herbal will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- digoxin
atorvastatin will increase the level or effect of digoxin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- diltiazem
diltiazem will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. If concurrent therapy is required, monitor for signs and symptoms of myopathy or rhabdomyolysis (muscle pain, tenderness, or weakness, or discolored urine). If myopathy or rhabdomyolysis is diagnosed or suspected, monitor creatine kinase (CK) levels and discontinue use if CK levels show a marked increase.
- docetaxel
atorvastatin will increase the level or effect of docetaxel by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- doxorubicin
atorvastatin will increase the level or effect of doxorubicin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- doxorubicin liposomal
atorvastatin will increase the level or effect of doxorubicin liposomal by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- dronedarone
dronedarone will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
dronedarone will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - duvelisib
duvelisib will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. will increase the level or effect of
- efavirenz
efavirenz will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- elacestrant
elacestrant will increase the level or effect of atorvastatin by Other (see comment). Modify Therapy/Monitor Closely. Elacestrant (a BCRP inhibitor) may increase plasma concentrations of sensitive BCRP substrates, which may increase risk of adverse reactions related to these substrates. Refer to prescribing information for sensitive BCRP substrates for dosing recommendations.
- elagolix
elagolix will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
elagolix will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Elagolix is a weak-to-moderate CYP3A4 inducer. Monitor CYP3A substrates if coadministered. Consider increasing CYP3A substrate dose if needed. - elbasvir/grazoprevir
elbasvir/grazoprevir increases levels of atorvastatin by unknown mechanism. Modify Therapy/Monitor Closely. If coadministered, do not exceed atorvastatin dose of 20 mg/day.
- eliglustat
eliglustat increases levels of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Modify Therapy/Monitor Closely. Monitor therapeutic drug concentrations, as indicated, or consider reducing the dosage of the P-gp substrate and titrate to clinical effect.
- eltrombopag
eltrombopag increases levels of atorvastatin by decreasing metabolism. Use Caution/Monitor. OATP transporter protein inhibition.
- elvitegravir/cobicistat/emtricitabine/tenofovir DF
elvitegravir/cobicistat/emtricitabine/tenofovir DF increases levels of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Cobicistat is a CYP3A4 inhibitor; initiate atorvastatin with the lowest starting dose and titrate carefully while monitoring for safety; do not exceed atorvastatin dose of 20 mg/day.
- encorafenib
encorafenib, atorvastatin. affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Encorafenib both inhibits and induces CYP3A4 at clinically relevant plasma concentrations. Coadministration of encorafenib with sensitive CYP3A4 substrates may result in increased toxicity or decreased efficacy of these agents.
encorafenib will increase the level or effect of atorvastatin by Other (see comment). Modify Therapy/Monitor Closely. Encorafenib (a OATP1B1, OATP1B3, and BCRP inhibitor) may increase the concentration and toxicities of OATP1B1, OATP1B3, and BCRP substrates. Closely monitor for signs and symptoms of increased exposure and consider adjusting the dose of these substrates. Screen reader support enabled. - erlotinib
atorvastatin will increase the level or effect of erlotinib by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- erythromycin base
erythromycin base will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- erythromycin ethylsuccinate
erythromycin ethylsuccinate will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- erythromycin lactobionate
erythromycin lactobionate will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- erythromycin stearate
erythromycin stearate will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- eslicarbazepine acetate
eslicarbazepine acetate will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- estradiol
atorvastatin will increase the level or effect of estradiol by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- estropipate
atorvastatin will increase the level or effect of estropipate by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- ethinylestradiol
atorvastatin will increase the level or effect of ethinylestradiol by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- etoposide
atorvastatin will increase the level or effect of etoposide by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- etravirine
etravirine will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- fedratinib
fedratinib will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Adjust dose of drugs that are CYP3A4 substrates as necessary.
- finerenone
atorvastatin will increase the level or effect of finerenone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Monitor serum potassium during initiation and dosage adjustment of either finererone or weak CYP3A4 inhibitors. Adjust finererone dosage as needed.
- flibanserin
atorvastatin will increase the level or effect of flibanserin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Increased flibanserin adverse effects may occur if coadministered with multiple weak CYP3A4 inhibitors.
- fluconazole
fluconazole will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- fludrocortisone
atorvastatin will increase the level or effect of fludrocortisone by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- fosaprepitant
fosaprepitant will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- fosphenytoin
fosphenytoin will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- fostamatinib
fostamatinib will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. Concomitant use of fostamatinib may increase concentrations of P-gp substrates. Monitor for toxicities of the P-gp substrate drug that may require dosage reduction when given concurrently with fostamatinib.
- fostemsavir
fostemsavir will increase the level or effect of atorvastatin by Other (see comment). Modify Therapy/Monitor Closely. Fostemsavir inhibits OATP1B1/3 and BCRP transporters. If possible, avoid coadministration or modify dose of OATP1B1/3 or BCRP substrates coadministered with fostemsavir. Use lowest possible starting dose for statins and monitor for associated adverse events.
- glyburide
glyburide increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- grapefruit
grapefruit will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- griseofulvin
griseofulvin will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- hydrocortisone
hydrocortisone will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
atorvastatin will increase the level or effect of hydrocortisone by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - iloperidone
iloperidone increases levels of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Iloperidone is a time-dependent CYP3A inhibitor and may lead to increased plasma levels of drugs predominantly eliminated by CYP3A4.
- imatinib
atorvastatin will increase the level or effect of imatinib by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- indinavir
atorvastatin will increase the level or effect of indinavir by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- irinotecan
atorvastatin will increase the level or effect of irinotecan by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- irinotecan liposomal
atorvastatin will increase the level or effect of irinotecan liposomal by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- isavuconazonium sulfate
atorvastatin will increase the level or effect of isavuconazonium sulfate by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- isoniazid
isoniazid will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- istradefylline
istradefylline will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Istradefylline 40 mg/day increased peak levels and AUC of CYP3A4 substrates in clinical trials. This effect was not observed with istradefylline 20 mg/day. Consider dose reduction of sensitive CYP3A4 substrates.
istradefylline will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. Istradefylline 40 mg/day increased peak levels and AUC of P-gp substrates in clinical trials. Consider dose reduction of sensitive P-gp substrates. - ivacaftor
ivacaftor increases levels of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. Ivacaftor and its M1 metabolite has the potential to inhibit P-gp; may significantly increase systemic exposure to sensitive P-gp substrates with a narrow therapeutic index.
- ivermectin
atorvastatin will increase the level or effect of ivermectin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- ketoconazole
ketoconazole will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Consider the risk/benefit of concomitant use of ketoconazole with atorvastatin. Monitor for signs and symptoms of myopathy particularly during initiation and dose titration of either drug.
ketoconazole will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - lanthanum carbonate
lanthanum carbonate decreases levels of atorvastatin by cation binding in GI tract. Use Caution/Monitor. Administer statin at least 2 hr before or 2 hr after lanthanum. Monitor serum concentrations.
- lapatinib
lapatinib will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
atorvastatin will increase the level or effect of lapatinib by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - ledipasvir/sofosbuvir
ledipasvir/sofosbuvir will increase the level or effect of atorvastatin by unspecified interaction mechanism. Modify Therapy/Monitor Closely. Coadministration of ledipasvir/sofosbuvir with atorvastatin may be associated withincreased risk of myopathy,including rhabdomyolysis. Monitor closely.
- lemborexant
atorvastatin will increase the level or effect of lemborexant by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Lower nightly dose of lemborexant recommended if coadministered with weak CYP3A4 inhibitors. See drug monograph for specific dosage modification.
- lenacapavir
lenacapavir will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Lencapavir (a moderate CYP3A4 inhibitor) may increase CYP3A4 substrates initiated within 9 months after last SC dose of lenacapavir, which may increase potential risk of adverse reactions of CYP3A4 substrates.
- letermovir
atorvastatin increases levels of letermovir by decreasing metabolism. Use Caution/Monitor. Coadminstration of letermovir, an OATP1B1/3 substrate, with OATP1B1/3 inhibitors may increase letermovir plasma concentrations.
letermovir increases levels of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Coadministration with atorvastatin and letermovir, do not exceed an atorvastatin dosage of 20 mg daily. Closely monitor patients for myopathy and rhabdomyolysis. When letermovir is coadministered with cyclosporine, use of atorvastatin is not recommended. . - levoketoconazole
levoketoconazole will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Consider the risk/benefit of concomitant use of ketoconazole with atorvastatin. Monitor for signs and symptoms of myopathy particularly during initiation and dose titration of either drug.
levoketoconazole will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - levonorgestrel oral/ethinylestradiol/ferrous bisglycinate
atorvastatin increases levels of levonorgestrel oral/ethinylestradiol/ferrous bisglycinate by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. Coadministration of atorvastatin and certain combined hormonal contraceptives (CHCs) containing EE increase AUC values for EE by approximately 20-25%.
- liraglutide
liraglutide will decrease the level or effect of atorvastatin by Other (see comment). Use Caution/Monitor. Based on pharmacokinetic studies liraglutide decreased atorvastatin Cmax by 38% and median Tmax delayed from 1h to 3h with liraglutide and the AUC did not change.
- lomitapide
atorvastatin increases levels of lomitapide by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Lomitapide dose should not exceed 30 mg/day.
lomitapide increases levels of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Modify Therapy/Monitor Closely. Consider reducing dose when used concomitantly with lomitapide. - loperamide
atorvastatin will increase the level or effect of loperamide by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- lorlatinib
lorlatinib will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- lovastatin
atorvastatin will increase the level or effect of lovastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- lumefantrine
lumefantrine will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- maraviroc
atorvastatin will increase the level or effect of maraviroc by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- marijuana
marijuana will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- mestranol
atorvastatin will increase the level or effect of mestranol by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- methylprednisolone
methylprednisolone will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
atorvastatin will increase the level or effect of methylprednisolone by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - metronidazole
metronidazole will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- metyrapone
metyrapone increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- miconazole vaginal
miconazole vaginal will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- midazolam intranasal
atorvastatin will increase the level or effect of midazolam intranasal by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Coadministration of mild CYP3A4 inhibitors with midazolam intranasal may cause higher midazolam systemic exposure, which may prolong sedation.
- mifepristone
mifepristone will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Dose of atorvastatin should not exceed 40 mg/day, when administered with a strong CYP3A4 inhibitor
- mipomersen
mipomersen increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- mitotane
mitotane decreases levels of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Mitotane is a strong inducer of cytochrome P-4503A4; monitor when coadministered with CYP3A4 substrates for possible dosage adjustments.
- momelotinib
momelotinib increases toxicity of atorvastatin by plasma protein binding competition. Modify Therapy/Monitor Closely. Momelotinib (BCRP inhibitor) may increase exposure of BCRP substrates, which may increase the risk of BCRP substrate adverse reactions. Dose adjustment of other BCRP substrates may necessary.
- naldemedine
atorvastatin increases levels of naldemedine by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. Monitor naldemedine for potential adverse effects if coadministered with P-gp inhibitors.
- nefazodone
nefazodone will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- nelfinavir
nelfinavir will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Limit atorvastatin dose to 40 mg/day
atorvastatin will increase the level or effect of nelfinavir by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - nevirapine
nevirapine will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- nicardipine
nicardipine will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- nifedipine
nifedipine will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
nifedipine will decrease the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - nilotinib
nilotinib will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
atorvastatin will increase the level or effect of nilotinib by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - nintedanib
atorvastatin increases effects of nintedanib by P-glycoprotein (MDR1) efflux transporter. Modify Therapy/Monitor Closely. If nintedanib adverse effects occur, management may require interruption, dose reduction, or discontinuation of therapy .
- nirmatrelvir
nirmatrelvir will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Consider temporary discontinuation of atorvastatin during treatment with nirmatrelvir/ritonavir.
- nirmatrelvir/ritonavir
nirmatrelvir/ritonavir will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Consider temporary discontinuation of atorvastatin during treatment with nirmatrelvir/ritonavir.
- oteseconazole
oteseconazole will increase the level or effect of atorvastatin by Other (see comment). Modify Therapy/Monitor Closely. Otesezonale, a BCRP inhibitor, may increase the effects and risk of toxicities of BCRP substrates. Use lowest starting dose of BCRP substrate, or consider reducing BCRP substrate dose.
- oxcarbazepine
oxcarbazepine will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- paclitaxel
atorvastatin will increase the level or effect of paclitaxel by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
paclitaxel increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy. - paclitaxel protein bound
atorvastatin will increase the level or effect of paclitaxel protein bound by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- paliperidone
atorvastatin will increase the level or effect of paliperidone by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- pazopanib
pazopanib increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- pentobarbital
pentobarbital will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- phenobarbital
phenobarbital will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
phenobarbital will decrease the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - phenytoin
phenytoin will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- pioglitazone
pioglitazone increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- ponatinib
ponatinib increases levels of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
ponatinib increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy. - posaconazole
posaconazole will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- prednisolone
atorvastatin will increase the level or effect of prednisolone by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- prednisone
prednisone will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
atorvastatin will increase the level or effect of prednisone by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - primidone
primidone will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- quercetin
quercetin will decrease the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- quinupristin/dalfopristin
quinupristin/dalfopristin will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- ranolazine
ranolazine will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
ranolazine increases toxicity of atorvastatin by Other (see comment). Modify Therapy/Monitor Closely. Comment: OATP1B1 inhibitors may increase risk of myopathy. - regorafenib
regorafenib will increase the level or effect of atorvastatin by Other (see comment). Use Caution/Monitor. Regorafenib likely inhibits BCRP (ABCG2) transport. Coadministration with a BCRP substrate may increase systemic exposure to the substrate and related toxicity
- repaglinide
repaglinide increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- resmetirom
resmetirom will increase the level or effect of atorvastatin by Other (see comment). Modify Therapy/Monitor Closely. Resmetirom inhibits BCRP and OAT1B1/3 transporters. Monitor for statin-related adverse reactions, including but not limited to elevation of liver tests, myopathy, and rhabdomyolysis. Limit daily dosage of statin as recommended.
- ribociclib
ribociclib will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- rifampin
rifampin will decrease the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- rifapentine
rifapentine will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- risperidone
atorvastatin will increase the level or effect of risperidone by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- ritonavir
ritonavir will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
atorvastatin will increase the level or effect of ritonavir by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - rosiglitazone
rosiglitazone increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- rucaparib
rucaparib will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Adjust dosage of CYP3A4 substrates, if clinically indicated.
- rufinamide
rufinamide will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- sacubitril/valsartan
sacubitril/valsartan increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy.
atorvastatin will increase the level or effect of sacubitril/valsartan by Other (see comment). Use Caution/Monitor. The results from an in vitro study with human liver tissue indicate that valsartan is a substrate of the hepatic uptake transporter OATP1B1; coadministration with OATP1B1 inhibitors may increase valsartan systemic exposure - safinamide
safinamide will increase the level or effect of atorvastatin by Other (see comment). Use Caution/Monitor. Safinamide and its major metabolite may inhibit intestinal BCRP. Monitor BCRP substrates for increased pharmacologic or adverse effects.
- saquinavir
atorvastatin will increase the level or effect of saquinavir by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- sarecycline
sarecycline will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. Monitor for toxicities of P-gp substrates that may require dosage reduction when coadministered with P-gp inhibitors.
- secobarbital
secobarbital will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- silodosin
atorvastatin will increase the level or effect of silodosin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- simvastatin
simvastatin will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- sirolimus
atorvastatin will increase the level or effect of sirolimus by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- sodium zirconium cyclosilicate
sodium zirconium cyclosilicate will increase the level or effect of atorvastatin by increasing gastric pH. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Separate administration by at least 2 hr. Medications that are weak acids (eg, atorvastatin) are more readily absorbed with elevated gastric pH.
- sofosbuvir/velpatasvir
sofosbuvir/velpatasvir increases levels of atorvastatin by Other (see comment). Modify Therapy/Monitor Closely. Comment: Velpatasvir is an inhibitor of OATP1B1, OATP1B3, and OATP2B1 transporters. Coadministration may increase systemic exposure of drugs that are substrates of these transporters. Coadministration may significantly increase atorvastatin serum concentration, which is associated with increased risk of myopathy, including rhabdomyolysis.
sofosbuvir/velpatasvir will increase the level or effect of atorvastatin by unspecified interaction mechanism. Modify Therapy/Monitor Closely. Coadministration of sofosbuvir/velpatasvir with atorvastatin may be associated withincreased risk of myopathy,including rhabdomyolysis. Monitor closely. - St John's Wort
St John's Wort will decrease the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- stiripentol
stiripentol, atorvastatin. affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Stiripentol is a CYP3A4 inhibitor and inducer. Monitor CYP3A4 substrates coadministered with stiripentol for increased or decreased effects. CYP3A4 substrates may require dosage adjustment.
stiripentol will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Modify Therapy/Monitor Closely. Consider reducing the dose of P-glycoprotein (P-gp) substrates, if adverse reactions are experienced when administered concomitantly with stiripentol.
stiripentol will increase the level or effect of atorvastatin by Other (see comment). Modify Therapy/Monitor Closely. Stiripentol is a BCRP transport inhibitor. Consider dosage reduction for BCRP substrates if adverse effects are experienced when coadministered. - tacrolimus
atorvastatin will increase the level or effect of tacrolimus by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
tacrolimus increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy. - tafamidis
tafamidis will increase the level or effect of atorvastatin by Other (see comment). Use Caution/Monitor. Tafamidis inhibits breast cancer resistant protein (BCRP) in vitro and may increase exposure of BCRP substrates following tafamidis or tafamidis meglumine administration. Dosage adjustment of these BCRP substrates may be necessary.
- tafamidis meglumine
tafamidis meglumine will increase the level or effect of atorvastatin by Other (see comment). Use Caution/Monitor. Tafamidis inhibits breast cancer resistant protein (BCRP) in vitro and may increase exposure of BCRP substrates following tafamidis or tafamidis meglumine administration. Dosage adjustment of these BCRP substrates may be necessary.
- tazemetostat
tazemetostat will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
atorvastatin will decrease the level or effect of tazemetostat by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. - tecovirimat
tecovirimat will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Tecovirimat is a weak CYP3A4 inducer. Monitor sensitive CYP3A4 substrates for effectiveness if coadministered.
- telmisartan
telmisartan increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy.
- tenapanor
tenapanor decreases levels of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: Tenapanor (an inhibitor of intestinal uptake transporter, OATP2B1) may reduce the exposure of OATP2B1 substrates.
- teniposide
atorvastatin will increase the level or effect of teniposide by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- tinidazole
atorvastatin will increase the level or effect of tinidazole by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- tolvaptan
atorvastatin will increase the level or effect of tolvaptan by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
tolvaptan will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - topiramate
topiramate will decrease the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- trazodone
trazodone will decrease the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- tucatinib
tucatinib will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. Consider reducing the dosage of P-gp substrates, where minimal concentration changes may lead to serious or life-threatening toxicities.
- vadadustat
vadadustat will increase the level or effect of atorvastatin by Other (see comment). Use Caution/Monitor. Vadadustat may increase exposure of BCRP substrates. Monitor for signs of adverse effect of BCRP substrate and reduce substrate dose in accordance with their product labeling.
- valsartan
atorvastatin will increase the level or effect of valsartan by Other (see comment). Use Caution/Monitor. The results from an in vitro study with human liver tissue indicate that valsartan is a substrate of the hepatic uptake transporter OATP1B1; coadministration with OATP1B1 inhibitors may increase valsartan systemic exposure
valsartan increases toxicity of atorvastatin by Other (see comment). Use Caution/Monitor. Comment: OATP1B1 inhibitors may increase risk of myopathy. - vemurafenib
vemurafenib increases levels of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- verapamil
verapamil will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
verapamil will increase the level or effect of atorvastatin by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. - vinblastine
atorvastatin will increase the level or effect of vinblastine by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- vincristine
atorvastatin will increase the level or effect of vincristine by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- vincristine liposomal
atorvastatin will increase the level or effect of vincristine liposomal by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor.
- voriconazole
voriconazole will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- zafirlukast
zafirlukast will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
Minor (17)
- acetazolamide
acetazolamide will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Minor/Significance Unknown.
- alvimopan
atorvastatin will increase the level or effect of alvimopan by P-glycoprotein (MDR1) efflux transporter. Minor/Significance Unknown.
- anastrozole
anastrozole will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Minor/Significance Unknown.
- armodafinil
atorvastatin will increase the level or effect of armodafinil by P-glycoprotein (MDR1) efflux transporter. Minor/Significance Unknown.
- coenzyme Q10
atorvastatin decreases levels of coenzyme Q10 by unspecified interaction mechanism. Minor/Significance Unknown.
- colestipol
colestipol decreases levels of atorvastatin by inhibition of GI absorption. Applies only to oral form of both agents. Minor/Significance Unknown.
- cyclophosphamide
cyclophosphamide will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Minor/Significance Unknown.
- drospirenone
drospirenone will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Minor/Significance Unknown.
- fexofenadine
atorvastatin will increase the level or effect of fexofenadine by P-glycoprotein (MDR1) efflux transporter. Minor/Significance Unknown.
- isradipine
isradipine decreases levels of atorvastatin by unknown mechanism. Minor/Significance Unknown.
- larotrectinib
larotrectinib will increase the level or effect of atorvastatin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Minor/Significance Unknown.
- loratadine
atorvastatin will increase the level or effect of loratadine by P-glycoprotein (MDR1) efflux transporter. Minor/Significance Unknown.
- orlistat
orlistat increases effects of atorvastatin by pharmacodynamic synergism. Minor/Significance Unknown.
- ruxolitinib
atorvastatin will increase the level or effect of ruxolitinib by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Minor/Significance Unknown.
- ruxolitinib topical
atorvastatin will increase the level or effect of ruxolitinib topical by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Minor/Significance Unknown.
- trazodone
trazodone increases levels of atorvastatin by unspecified interaction mechanism. Minor/Significance Unknown.
- voclosporin
voclosporin will increase the level or effect of atorvastatin by Other (see comment). Minor/Significance Unknown. Information suggests voclosporin (an OATP1B1 inhibitor) may increase in the concentration of OATP1B1 substrates is possible. Monitor for adverse reactions of OATP1B1 substrates when coadministered with voclosporin.
Adverse Effects
>10%
Diarrhea (5-14%)
Nasopharyngitis (4-13%)
Arthralgia (4-12%)
1-10%
Insomnia (1-5%)
Urinary tract infection (4-8%)
Nausea (4-7%)
Dyspepsia (3-6%)
Increased transaminases (2-3%)
Muscle spasms (2-5%)
Musculoskeletal pain (2-5%)
Myalgia (3-8%)
Limb pain (3-8%)
Pharyngolaryngeal pain (1-4%)
Frequency Not Defined
Angina
Syncope
Dyspnea
Myopathy
Anaphylaxis
Stevens-Johnson syndrome
Myositis
Postmarketing Reports
New-onset or exacerbation of myasthenia gravis, including ocular myasthenia, and reports of recurrence when same or different statin administered
Warnings
Contraindications
Hypersensitivity to atorvastatin
Acute liver failure or decompensated cirrhosis
Cautions
Nonserious and reversible cognitive side effects may occur
Fatal and nonfatal hepatic failure reported (rare)
Hypersensitivity reactions, including anaphylaxis, angioneurotic edema, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis, reported
Increases in HbA1c and fasting serum glucose levels reported with therapy; optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices
Renal impairment is a risk factor for myopathy and rhabdomyolysis; monitor all patients with renal impairment for development of myopathy; renal impairment does not affect the plasma concentrations of this drug; thus there is no dosage adjustment in patients with renal impairment
In patients with chronic alcoholic liver disease, plasma concentrations of this drug are markedly increased; Cmax and AUC are each 4-fold greater in patients with Childs-Pugh A disease; Cmax and AUC are approximately 16-fold and 11-fold increased, respectively, in patients with Childs-Pugh B disease; this drug is contraindicated in patients with acute liver failure or decompensated cirrhosis
Hepatic dysfunction
- Increases in serum transaminases reported with therapy; in most cases, these changes appeared soon after initiation, were transient, were not accompanied by symptoms, and resolved or improved on continued therapy or after a brief interruption in therapy
- Persistent increases to more than three times the ULN in serum transaminases have occurred in approximately 0.7% of patients receiving LIPITOR in clinical trials
- There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins
- Patients who consume substantial quantities of alcohol and/or have a history of liver disease may be at increased risk for hepatic injury
- Consider liver enzyme testing before initiating therapy and when clinically indicated thereafter
- Therapy is contraindicated in patients with acute liver failure or decompensated cirrhosis; if serious hepatic injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs, promptly discontinue therapy
Myopathy and rhabdomyolysis
- Risk factors for myopathy include age 65 years or greater, uncontrolled hypothyroidism, renal impairment, concomitant use with certain other drugs, and higher dosage
- Therapy may cause myopathy (muscle pain, tenderness, or weakness with creatine kinase (CK) above ten times upper limit of normal) and rhabdomyolysis (with or without acute renal failure secondary to myoglobinuria); rare fatalities have occurred as a result of rhabdomyolysis with statin use
- Risk of myopathy: Increased by coadministration with fibrates, cyclosporine, macrolides, inhibition of cytochrome P450 enzyme 3A4 (CYP3A4) and/or transporters (eg, breast cancer resistant protein [BCRP], organic anion-transporting polypeptide [OATP1B1/OATP1B3] and P-glycoprotein [P-gp]), resulting in an increased risk of myopathy and rhabdomyolysis; concomitant use of cyclosporine, gemfibrozil, tipranavir plus ritonavir, or glecaprevir plus pibrentasvir not recommended
- Cases of myopathy/rhabdomyolysis reported with atorvastatin coadministered with lipid modifying doses (>1 gram/day) of niacin, fibrates, colchicine, and ledipasvir plus sofosbuvir; consider if benefit of use of these products outweighs increased risk of myopathy and rhabdomyolysis
- Atorvastatin dosage modifications recommended for patients taking certain anti-viral, azole antifungals, or macrolide antibiotic medications
- Withhold or discontinue treatment in any patient developing myopathy, renal failure, or transaminase levels >3x ULN
- Temporary therapy discontinuation recommended for patients with acute surgical or medical conditions, elective major surgery, or serious condition suggestive of a myopathy or risk factor predisposing to development of renal failure secondary to rhabdomyolysis
- Discontinue therapy if markedly elevated CK levels occur or myopathy is diagnosed or suspected
- Use caution in hepatic impairment, recent stroke
- CYP3A4 substrate; avoid grapefruit products and caution with other CYP3A4 inhibitors; concomitant intake of large quantities, more than 1.2 liters daily, of grapefruit juice not recommended
- Muscle symptoms and CK increases may resolve if atorvastatin is discontinued; temporarily discontinue therapy in patients experiencing acute or serious condition at high risk of developing renal failure secondary to rhabdomyolysis (eg, sepsis; shock; severe hypovolemia; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy)
- Secondary causes of hyperlipidemia should be ruled out before initiating therapy
Immune-mediated necrotizing myopathy
- Immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, reported with statin use
- IMNM is characterized by muscle biopsy showing necrotizing myopathy without significant inflammation improvement with immunosuppressive agents, proximal muscle weakness, and elevated serum creatine kinase, which persist despite discontinuation of statin treatment
- Treatment with immunosuppressive agents may be required
- Advice all patients starting therapy or whose dose is being increased, about the risk of myopathy, including rhabdomyolysis
- Patients should report promptly any unexplained muscle pain, tenderness, or weakness particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing therapy; additional neuromuscular and serologic testing may be necessary
- Therapy should be discontinued immediately if myopathy is diagnosed or suspected
- Discontinue therapy if markedly elevated creatine kinase (CK) levels occur or if myopathy diagnosed or suspected
- Therapy should be temporarily withheld in any patient experiencing an acute or serious condition predisposing to development of renal failure secondary to rhabdomyolysis, eg, sepsis; hypotension; dehydration; major surgery; trauma; severe metabolic, endocrine, and electrolyte disorders; or uncontrolled epilepsy
- Consider risk of IMNM carefully prior to initiation of a different statin
- If therapy is initiated with a different statin, monitor for signs and symptoms of IMNM
- Additional neuromuscular and serologic testing may be necessary
- Treatment with immunosuppressive agents may be required
- Consider risk of IMNM carefully prior to initiation of a different statin
- If therapy is initiated with a different statin, monitor for signs and symptoms of IMNM
Pregnancy & Lactation
Pregnancy
Owing to HMG-CoA reductase inhibitors decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, fetal harm may occur when administered to pregnant females; discontinue therapy as soon as pregnancy is recognized
Treatment of hyperlipidemia is not generally necessary during pregnancy; atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hyperlipidemia for most patients
Available data from case series and prospective and retrospective observational cohort studies over decades of use with statins in pregnant women have not identified a drug-associated risk of major congenital malformations; published data from prospective and retrospective observational cohort studies with use in pregnant women are insufficient to determine if there is a drug-associated risk of miscarriage
Contraception
- Females of reproductive potential: Use effective contraception during treatment
Animal data
- In animal reproduction studies, no adverse developmental effects were observed in pregnant rats or rabbits orally administered atorvastatin at doses that resulted in up to 30 and 20 times, respectively, the human exposure at the maximum recommended human dose (MRHD) of 80 mg, based on body surface area (mg/m2); in rats administered atorvastatin during gestation and lactation, decreased postnatal growth and development delay were observed at doses greater than or equal to 6 times the MRHD
FDA MedWatch
- On July 20, 2021, the FDA request to remove the contraindication against HMG-CoA reductase inhibitors in pregnant females
- Despite the changes, most females found to be pregnant should stop therapy
Lactation
There is no available information on effects of drug on breastfed infant or on milk production
Unknown whether is present in human milk; it has been shown that drugs in this class pass into human milk and atorvastatin is present in rat milk
Studies in rats have shown that atorvastatin and/or its metabolites are present in the breast milk of lactating rats; when a drug is present in animal milk, it is likely that the drug will be present in human milk; this drug decreases cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol and may cause harm to breastfed infant
Because of potential for serious adverse reactions in a breastfed infant, based on mechanism of action, advise patients that breastfeeding is not recommended during therapy
FDA MedWatch
- On July 20, 2021, the FDA request to remove the contraindication against HMG-CoA reductase inhibitors in pregnant females
- Breastfeeding is still not recommended if taking statins; drug may still pass through milk and pose a risk breastfed children
- For patients with lower risk, temporarily stop statin therapy until breastfeeding ends
- Patients who are at high risk of heart attack or stroke who require statins after delivery should not breastfeed and should use alternatives such as infant formula
Pregnancy Categories
A: Generally acceptable. Controlled studies in pregnant women show no evidence of fetal risk.
B: May be acceptable. Either animal studies show no risk but human studies not available or animal studies showed minor risks and human studies done and showed no risk. C: Use with caution if benefits outweigh risks. Animal studies show risk and human studies not available or neither animal nor human studies done. D: Use in LIFE-THREATENING emergencies when no safer drug available. Positive evidence of human fetal risk. X: Do not use in pregnancy. Risks involved outweigh potential benefits. Safer alternatives exist. NA: Information not available.Pharmacology
Mechanism of Action
HMG-CoA reductase inhibitor; inhibits rate-limiting step in cholesterol biosynthesis by competitively inhibiting HMG-CoA reductase
Absorption
Bioavailability: 14% (parent drug)
Onset: 3-5 days
Duration: 48-72 hr
Peak serum time: 1-2 hr
Maximum effect: 2 weeks
Distribution
Protein bound: 98%
Vd: 381 L
Metabolism
Via hepatic P450 enzyme CYP3A4
Metabolites: Ortho- and parahydroxylated derivatives and beta-oxidation product (inactive)
Elimination
Half-life: 14 hr
Dialyzable: No (HD)
Excretion: Mainly via bile; urine (2%)
Pharmacogenomics
SLCO1B1 (OATP1B1) CC genotype significantly increases AUCs of parent drug and metabolites compared with the CT or TT genotypes
This polymorphism is proposed to reduce transport into the liver, the main site of statin metabolism and elimination
SLCO1B1 polymorphism is thought to have a lesser effect on the more hydrophilic statins (eg, rosuvastatin, fluvastatin) compared with those that are more lipophilic (eg, atorvastatin, simvastatin)
Other genetic polymorphisms of elimination (eg, CYP450, P-glycoprotein) for each individual drug must also be considered, to explain variability for statin clearance among patients that exhibit SCLO1B1 polymorphism
SLCO1B1 CC genotype is most common in Caucasians and Asians (15%)
Risk of myopathy is 2.6- to 4.3-fold higher if the C allele is present and 16.9-fold higher in CC homozygotes than in TT homozygotes
Genetic testing laboratories
- Optivia Biotechnology, Inc (http://optiviabio.com)
Administration
Oral Administration
Take once daily at any time of the day
Tablet
- May take with or without food
Oral suspension
- Take only on an empty stomach (1 hr before or 2 hr after a meal)
- Measure dose using calibrated oral syringe or other oral dosing device scored using metric units of measurements (ie, mL)
Missed dose
- Take as soon as possible
- If dose missed by >12 hours, do not take; wait until next scheduled dose
- Do not take 2 doses at the same time
Storage
Tablet: Store at 20-25ºC (68-77ºF)
Oral suspension
- Store at 20-25ºC (68-77ºF); excursions permitted to 15-30ºC (59-86ºF)
- Store and dispense in original bottle; use within 60 days of first opening bottle, then discard any remainder
Images
| BRAND | FORM. | UNIT PRICE | PILL IMAGE |
|---|---|---|---|
| Lipitor oral - | 80 mg tablet |  | |
| Lipitor oral - | 20 mg tablet |  | |
| Lipitor oral - | 10 mg tablet |  | |
| Lipitor oral - | 80 mg tablet |  | |
| Lipitor oral - | 20 mg tablet |  | |
| Lipitor oral - | 40 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 80 mg tablet | 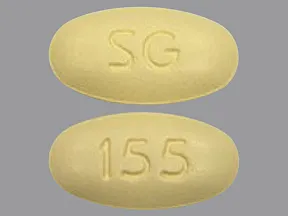 | |
| Atorvastatin - | 10 mg tablet | 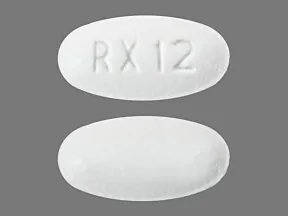 | |
| Atorvastatin - | 20 mg tablet | 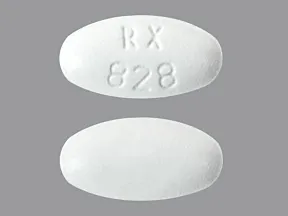 | |
| Atorvastatin - | 80 mg tablet | 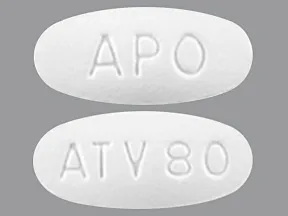 | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 20 mg tablet |  | |
| Atorvastatin - | 10 mg tablet |  | |
| Atorvastatin - | 80 mg tablet |  | |
| Atorvastatin - | 40 mg tablet |  |
Copyright © 2010 First DataBank, Inc.
Patient Handout
Atorvastatin
ATORVASTATIN SUSPENSION - ORAL
(a-TOR-va-STAT-in)
COMMON BRAND NAME(S): Atorvaliq
USES: Atorvastatin is used along with a proper diet to help lower "bad" cholesterol and fats (such as LDL, triglycerides) and raise "good" cholesterol (HDL) in the blood. It belongs to a group of drugs known as "statins." It works by reducing the amount of cholesterol made by the liver. Lowering "bad" cholesterol and triglycerides and raising "good" cholesterol decreases the risk of heart disease and helps prevent strokes and heart attacks.In addition to eating a proper diet (such as a low-cholesterol/low-fat diet), other lifestyle changes that may help this medication work better include exercising, losing weight if overweight, and stopping smoking. Consult your doctor for more details.
HOW TO USE: Read the Patient Information Leaflet if available from your pharmacist before you start taking atorvastatin and each time you get a refill. If you have any questions, ask your doctor or pharmacist.Take this medication by mouth as directed by your doctor, usually once daily. It should be taken on an empty stomach, 1 hour before or 2 hours after a meal.The dosage is based on your medical condition, response to treatment, age, and other medications you may be taking. Be sure to tell your doctor and pharmacist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).Shake the bottle well before each dose. Carefully measure the dose using a special measuring device/spoon. Do not use a household spoon because you may not get the correct dose.Avoid eating grapefruit or drinking grapefruit juice while using this medication unless your doctor or pharmacist says you may do so safely. Grapefruit can increase the chance of side effects with this medicine. Ask your doctor or pharmacist for more details.If you also take certain other drugs to lower your cholesterol (bile acid-binding resins such as cholestyramine or colestipol), take atorvastatin at least 1 hour before or at least 4 hours after taking these medications. These products can react with atorvastatin, preventing its full absorption.Take this medication regularly in order to get the most benefit from it. Remember to take it at the same time each day. Keep taking this medication even if you feel well. Most people with high cholesterol or triglycerides do not feel sick.It is very important to continue to follow your doctor's advice about diet and exercise. It may take up to 4 weeks before you get the full benefit of this drug.
SIDE EFFECTS: Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.A very small number of people taking atorvastatin may have mild memory problems or confusion. If these rare effects occur, talk to your doctor.Rarely, statins may cause or worsen diabetes. Talk to your doctor about the benefits and risks.This drug may rarely cause muscle problems (which can rarely lead to very serious conditions called rhabdomyolysis and autoimmune myopathy). Tell your doctor right away if you develop any of these symptoms during treatment and if these symptoms last after your doctor stops this drug: muscle pain/tenderness/weakness (especially with fever or unusual tiredness), signs of kidney problems (such as change in the amount of urine).This medication may rarely cause liver problems. Tell your doctor right away if you develop symptoms of liver problems, including: nausea/vomiting that doesn't stop, yellowing eyes/skin, dark urine, stomach/abdominal pain.A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.In the US -Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
PRECAUTIONS: Before taking atorvastatin, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.Before using this medication, tell your doctor or pharmacist your medical history, especially of: liver disease, kidney disease, alcohol use.Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).Limit alcoholic beverages. Daily use of alcohol may increase your risk for liver problems, especially when combined with atorvastatin. Ask your doctor or pharmacist for more information.Older adults may be more sensitive to the side effects of this drug, especially muscle problems.During pregnancy, this medication should be used only when clearly needed. It may harm an unborn baby. Discuss the risks and benefits with your doctor.It is unknown if this medication passes into breast milk. Because of the possible risk to the infant, breastfeeding is not recommended while using this medication. Consult your doctor before breastfeeding.
DRUG INTERACTIONS: See also How to Use section.Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.Some products that may interact with this drug include: daptomycin, gemfibrozil.Other medications can affect the removal of atorvastatin from your body, which may affect how atorvastatin works. Examples include glecaprevir plus pibrentasvir, telithromycin, among others.Do not take any red yeast rice products while you are taking atorvastatin because some red yeast rice products may also contain a statin called lovastatin. Taking atorvastatin and red yeast rice products together can increase your risk of serious muscle and liver problems.
OVERDOSE: If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center.
NOTES: Do not share this medication with others.Lab and/or medical tests (such as blood cholesterol/triglyceride levels, liver function) should be done while you are taking this medication. Keep all medical and lab appointments. Consult your doctor for more details.
MISSED DOSE: If you miss a dose, take it as soon as you remember. If it is more than 12 hours after the missed dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
STORAGE: Store at room temperature away from light and moisture. Do not store in the bathroom. Store the suspension form in the original container. Discard the suspension form 60 days after opening the bottle, even if there is medication left. Keep all medications away from children and pets.Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Information last revised February 2024. Copyright(c) 2024 First Databank, Inc.
IMPORTANT: HOW TO USE THIS INFORMATION: This is a summary and does NOT have all possible information about this product. This information does not assure that this product is safe, effective, or appropriate for you. This information is not individual medical advice and does not substitute for the advice of your health care professional. Always ask your health care professional for complete information about this product and your specific health needs.
Formulary
Adding plans allows you to compare formulary status to other drugs in the same class.
To view formulary information first create a list of plans. Your list will be saved and can be edited at any time.
Adding plans allows you to:
- View the formulary and any restrictions for each plan.
- Manage and view all your plans together – even plans in different states.
- Compare formulary status to other drugs in the same class.
- Access your plan list on any device – mobile or desktop.







