Dosing & Uses
Dosage Forms & Strengths
tablet
- 5mg
Congestive Heart Failure
5-10 mg/day PO qDay OR divided q12hr
Hypertension
5-10 mg/day PO qDay OR divided q12hr
Thiazide-Induced Hypokalemia
5-10 mg/day PO qDay OR divided q12hr
Renal Impairment
CrCl 10-50 mL/minute: 50% normal dose
CrCl <10 mL/minute: Not recommended
Overdose Management
May use normal saline for volume replacement
May use dopamine or norepinephrine to treat hypotension
Treat hyperkalemia with IV glucose (dextrose 25% in water) with rapid acting insulin with concurrent IV sodium bicarbonate and use oral or rectal solutions of kayexalate in sorbitol if needed
If dysrhythmia due to decreased K+ or Mg+ suspected replace aggressively
Discontinue treatment if no symptoms after 6hr
Other Information
Take with food
Monitor: Serum potassium
See also combo with hydrochlorothiazide
Other Indications & Uses
Off-label: cystic fibrosis, Li-induced polyuria
Dosage Forms & Strengths
tablet
- 5mg
Hypertension (Off-label)
0.4-0.625 mg/kg/day PO; not to exceed 20 mg/day
5-10 mg/day PO qDay OR every other day
Maximum dosage limit: 20 mg PO qDay; 40 mg PO qDay has been used
Elderly patients may be more sensitive to the diuretic effects of the drug and are more likely to have age-associated renal dysfunction
Interactions
Interaction Checker
No Results

Contraindicated
Serious - Use Alternative
Significant - Monitor Closely
Minor

Contraindicated (0)
Serious - Use Alternative (11)
- cyclosporine
amiloride and cyclosporine both increase serum potassium. Avoid or Use Alternate Drug. Coadministration not recommended
- drospirenone
amiloride and drospirenone both increase serum potassium. Avoid or Use Alternate Drug.
amiloride, drospirenone. Either increases effects of the other by pharmacodynamic synergism. Contraindicated. Hyperkalemia. - eplerenone
amiloride, eplerenone. Mechanism: pharmacodynamic synergism. Contraindicated. Hyperkalemia.
- lofexidine
lofexidine, amiloride. Either increases effects of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Avoid coadministration with other drugs that decrease pulse or blood pressure to mitigate risk of excessive bradycardia and hypotension.
- potassium acid phosphate
amiloride and potassium acid phosphate both increase serum potassium. Avoid or Use Alternate Drug.
- potassium chloride
amiloride and potassium chloride both increase serum potassium. Avoid or Use Alternate Drug.
- potassium phosphates, IV
amiloride and potassium phosphates, IV both increase serum potassium. Avoid or Use Alternate Drug.
- spironolactone
amiloride and spironolactone both increase serum potassium. Avoid or Use Alternate Drug.
amiloride, spironolactone. Either increases effects of the other by pharmacodynamic synergism. Contraindicated. Hyperkalemia. - tafenoquine
tafenoquine will increase the level or effect of amiloride by Other (see comment). Avoid or Use Alternate Drug. Tafenoquine inhibits organic cation transporter-2 (OCT2) and multidrug and toxin extrusion (MATE) transporters in vitro. Avoid coadministration with OCT2 or MATE substrates. If coadministration cannot be avoided, monitor for substrate-related toxicities and consider dosage reduction if needed based on product labeling of the coadministered drug.
- triamterene
amiloride and triamterene both increase serum potassium. Avoid or Use Alternate Drug.
amiloride, triamterene. Either increases effects of the other by pharmacodynamic synergism. Contraindicated. Hyperkalemia. - trilaciclib
trilaciclib will increase the level or effect of amiloride by Other (see comment). Avoid or Use Alternate Drug. Avoid coadministration of trilaciclib (OCT2, MATE1, and MATE-2K inhibitor) with substrates where minimal increased concentration in kidney or blood may lead to serious or life-threatening toxicities.
Monitor Closely (145)
- acebutolol
acebutolol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- aceclofenac
amiloride and aceclofenac both increase serum potassium. Modify Therapy/Monitor Closely.
- acemetacin
amiloride and acemetacin both increase serum potassium. Modify Therapy/Monitor Closely.
- albuterol
amiloride increases and albuterol decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- aldesleukin
aldesleukin increases effects of amiloride by pharmacodynamic synergism. Use Caution/Monitor. Risk of hypotension.
- amifostine
amifostine, amiloride. Either increases effects of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Coadministration with blood pressure lowering agents may increase the risk and severity of hypotension associated with amifostine. When amifostine is used at chemotherapeutic doses, withhold blood pressure lowering medications for 24 hr prior to amifostine; if blood pressure lowering medication cannot be withheld, do not administer amifostine.
- arformoterol
amiloride increases and arformoterol decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- aspirin
amiloride and aspirin both increase serum potassium. Modify Therapy/Monitor Closely.
- aspirin rectal
amiloride and aspirin rectal both increase serum potassium. Modify Therapy/Monitor Closely.
- aspirin/citric acid/sodium bicarbonate
amiloride and aspirin/citric acid/sodium bicarbonate both increase serum potassium. Modify Therapy/Monitor Closely.
- atenolol
atenolol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- avanafil
avanafil increases effects of amiloride by pharmacodynamic synergism. Use Caution/Monitor. Risk of hypotension.
- benazepril
benazepril and amiloride both increase serum potassium. Use Caution/Monitor. Risk of hyperkalemia. Enhanced hypotensive effects.
- bendroflumethiazide
amiloride increases and bendroflumethiazide decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- betaxolol
betaxolol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- bisoprolol
bisoprolol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- bretylium
amiloride, bretylium. Either increases effects of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Each drug may cause hypotension.
- bumetanide
amiloride increases and bumetanide decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- buprenorphine, long-acting injection
buprenorphine, long-acting injection decreases effects of amiloride by pharmacodynamic antagonism. Modify Therapy/Monitor Closely. Opioids can reduce diuretic efficacy by inducing antidiuretic hormone release.
- canagliflozin
amiloride and canagliflozin both increase serum potassium. Use Caution/Monitor.
- candesartan
candesartan and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- captopril
captopril, amiloride. Either increases toxicity of the other by Mechanism: pharmacodynamic synergism. Use Caution/Monitor. Risk of hyperkalemia. Both drugs lower blood pressure. Monitor potassium and blood pressure.
- carbenoxolone
amiloride increases and carbenoxolone decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- carbidopa
carbidopa increases effects of amiloride by pharmacodynamic synergism. Use Caution/Monitor. Therapy with carbidopa, given with or without levodopa or carbidopa-levodopa combination products, is started, dosage adjustment of the antihypertensive drug may be required.
- carvedilol
carvedilol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- celecoxib
amiloride and celecoxib both increase serum potassium. Modify Therapy/Monitor Closely.
- celiprolol
celiprolol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- chlorothiazide
amiloride increases and chlorothiazide decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- chlorthalidone
amiloride increases and chlorthalidone decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- choline magnesium trisalicylate
amiloride and choline magnesium trisalicylate both increase serum potassium. Modify Therapy/Monitor Closely.
- cyclopenthiazide
amiloride increases and cyclopenthiazide decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- dalteparin
amiloride, dalteparin. Either increases toxicity of the other by serum potassium. Use Caution/Monitor. Both drugs may increase serum potassium levels.
- dichlorphenamide
dichlorphenamide, amiloride. Either increases toxicity of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Both drugs can cause metabolic acidosis.
- diclofenac
amiloride and diclofenac both increase serum potassium. Modify Therapy/Monitor Closely.
- diflunisal
amiloride and diflunisal both increase serum potassium. Modify Therapy/Monitor Closely.
- digoxin
amiloride and digoxin both increase serum potassium. Modify Therapy/Monitor Closely.
- disopyramide
amiloride increases effects of disopyramide by pharmacodynamic synergism. Use Caution/Monitor. Additive cardiovascular depression.
- dobutamine
amiloride increases and dobutamine decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- dopexamine
amiloride increases and dopexamine decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- empagliflozin
empagliflozin, amiloride. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Coadministration of empagliflozin with diuretics results in increased urine volume and frequency of voids, which might enhance the potential for volume depletion.
- enalapril
enalapril, amiloride. Mechanism: pharmacodynamic synergism. Use Caution/Monitor. Risk of hyperkalemia.
- enoxaparin
amiloride, enoxaparin. Either increases toxicity of the other by serum potassium. Use Caution/Monitor. Both drugs may increase serum potassium levels.
- ephedrine
amiloride increases and ephedrine decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- epinephrine
amiloride increases and epinephrine decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- epinephrine racemic
amiloride increases and epinephrine racemic decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- eprosartan
eprosartan and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- erdafitinib
erdafitinib increases levels of amiloride by decreasing renal clearance. Modify Therapy/Monitor Closely. Consider alternatives that are not OCT2 substrates or consider reducing the dose of OCT2 substrates based on tolerability.
- esmolol
esmolol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- ethacrynic acid
amiloride increases and ethacrynic acid decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- etodolac
amiloride and etodolac both increase serum potassium. Modify Therapy/Monitor Closely.
- fenbufen
amiloride and fenbufen both increase serum potassium. Modify Therapy/Monitor Closely.
- fenoprofen
amiloride and fenoprofen both increase serum potassium. Modify Therapy/Monitor Closely.
- fentanyl
fentanyl decreases effects of amiloride by Other (see comment). Modify Therapy/Monitor Closely. Comment: Fentanyl can reduce the efficacy of diuretics by inducing antidiuretic hormone release. Fentanyl may also lead to acute urinary retention by causing bladder sphincter spasm (particularly in men with enlarged prostates).
- fentanyl intranasal
fentanyl intranasal decreases effects of amiloride by Other (see comment). Modify Therapy/Monitor Closely. Comment: Fentanyl can reduce the efficacy of diuretics by inducing antidiuretic hormone release. Fentanyl may also lead to acute urinary retention by causing bladder sphincter spasm (particularly in men with enlarged prostates).
- fentanyl transdermal
fentanyl transdermal decreases effects of amiloride by Other (see comment). Modify Therapy/Monitor Closely. Comment: Fentanyl can reduce the efficacy of diuretics by inducing antidiuretic hormone release. Fentanyl may also lead to acute urinary retention by causing bladder sphincter spasm (particularly in men with enlarged prostates).
- fentanyl transmucosal
fentanyl transmucosal decreases effects of amiloride by Other (see comment). Modify Therapy/Monitor Closely. Comment: Fentanyl can reduce the efficacy of diuretics by inducing antidiuretic hormone release. Fentanyl may also lead to acute urinary retention by causing bladder sphincter spasm (particularly in men with enlarged prostates).
- finerenone
amiloride and finerenone both increase serum potassium. Modify Therapy/Monitor Closely. Finerenone dose adjustment based on current serum potassium concentration. Monitor serum potassium and adjust finerenone dose as described in the prescribing information as necessary.
- flurbiprofen
amiloride and flurbiprofen both increase serum potassium. Modify Therapy/Monitor Closely.
- formoterol
amiloride increases and formoterol decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- fosinopril
fosinopril, amiloride. Mechanism: pharmacodynamic synergism. Use Caution/Monitor. Risk of hyperkalemia.
- furosemide
amiloride increases and furosemide decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- gentamicin
amiloride increases and gentamicin decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- givinostat
givinostat will increase the level or effect of amiloride by Other (see comment). Use Caution/Monitor. Givinostat is a weak OAT2 inhibitor. Closely monitor if coadministered with orally administered OCT2 sensitive substrates for which a small change in substrate plasma concentration may lead to serious toxicities.
- heparin
amiloride, heparin. Either increases toxicity of the other by serum potassium. Use Caution/Monitor. Both drugs may increase serum potassium levels.
- hydrochlorothiazide
amiloride increases and hydrochlorothiazide decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- ibuprofen
amiloride and ibuprofen both increase serum potassium. Modify Therapy/Monitor Closely.
- ibuprofen IV
amiloride and ibuprofen IV both increase serum potassium. Modify Therapy/Monitor Closely.
- imidapril
imidapril, amiloride. Mechanism: pharmacodynamic synergism. Use Caution/Monitor. Risk of hyperkalemia.
- indapamide
amiloride increases and indapamide decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- indomethacin
amiloride and indomethacin both increase serum potassium. Modify Therapy/Monitor Closely.
- irbesartan
irbesartan and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- isoproterenol
amiloride increases and isoproterenol decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- juniper
juniper, amiloride. Other (see comment). Use Caution/Monitor. Comment: Juniper may potentiate or interfere with diuretic therapy. Juniper has diuretic effects, but may cause kidney damage at large doses.
- ketoprofen
amiloride and ketoprofen both increase serum potassium. Modify Therapy/Monitor Closely.
- ketorolac
amiloride and ketorolac both increase serum potassium. Modify Therapy/Monitor Closely.
- ketorolac intranasal
amiloride and ketorolac intranasal both increase serum potassium. Modify Therapy/Monitor Closely.
- labetalol
labetalol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- levalbuterol
amiloride increases and levalbuterol decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- levodopa
levodopa increases effects of amiloride by pharmacodynamic synergism. Use Caution/Monitor. Consider decreasing dosage of antihypertensive agent.
- lisinopril
lisinopril, amiloride. Mechanism: pharmacodynamic synergism. Use Caution/Monitor. Risk of hyperkalemia.
- lornoxicam
amiloride and lornoxicam both increase serum potassium. Modify Therapy/Monitor Closely.
- losartan
losartan and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- lurasidone
lurasidone increases effects of amiloride by Other (see comment). Use Caution/Monitor. Comment: Potential for increased risk of hypotension with concurrent use. Monitor blood pressure and adjust dose of antihypertensive agent as needed.
- maraviroc
maraviroc, amiloride. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of orthostatic hypotension.
- meclofenamate
amiloride and meclofenamate both increase serum potassium. Modify Therapy/Monitor Closely.
- mefenamic acid
amiloride and mefenamic acid both increase serum potassium. Modify Therapy/Monitor Closely.
- meloxicam
amiloride and meloxicam both increase serum potassium. Modify Therapy/Monitor Closely.
- metaproterenol
amiloride increases and metaproterenol decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- methyclothiazide
amiloride increases and methyclothiazide decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely. .
- methylphenidate transdermal
methylphenidate transdermal decreases effects of amiloride by anti-hypertensive channel blocking. Use Caution/Monitor.
- metolazone
amiloride increases and metolazone decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- metoprolol
metoprolol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- moexipril
moexipril, amiloride. Mechanism: pharmacodynamic synergism. Use Caution/Monitor. Risk of hyperkalemia.
- nabumetone
amiloride and nabumetone both increase serum potassium. Modify Therapy/Monitor Closely.
- nadolol
nadolol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- naproxen
amiloride and naproxen both increase serum potassium. Modify Therapy/Monitor Closely.
- nebivolol
nebivolol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- nitroglycerin rectal
nitroglycerin rectal, amiloride. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Observe for possible additive hypotensive effects during concomitant use. .
- noni juice
amiloride and noni juice both increase serum potassium. Use Caution/Monitor.
- norepinephrine
amiloride increases and norepinephrine decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- olanzapine/samidorphan
olanzapine/samidorphan increases effects of amiloride by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Monitor blood pressure and reduce antihypertensive drug dosage in accordance with its product labeling.
- oliceridine
oliceridine decreases effects of amiloride by Other (see comment). Use Caution/Monitor. Comment: Opioids can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone. Monitor for signs of diminished diuresis and/or effects on blood pressure and increase dosage of the diuretic as needed. .
- olmesartan
olmesartan and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- oxaprozin
amiloride and oxaprozin both increase serum potassium. Modify Therapy/Monitor Closely.
- parecoxib
amiloride and parecoxib both increase serum potassium. Modify Therapy/Monitor Closely.
- penbutolol
penbutolol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- penicillin G aqueous
penicillin G aqueous increases effects of amiloride by unspecified interaction mechanism. Use Caution/Monitor. Hyperkalemia.
- perindopril
perindopril, amiloride. Mechanism: pharmacodynamic synergism. Use Caution/Monitor. Risk of hyperkalemia.
- pindolol
pindolol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- pirbuterol
amiloride increases and pirbuterol decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- piroxicam
amiloride and piroxicam both increase serum potassium. Modify Therapy/Monitor Closely.
- pivmecillinam
pivmecillinam increases effects of amiloride by unspecified interaction mechanism. Use Caution/Monitor. Hyperkalemia.
- potassium citrate
amiloride and potassium citrate both increase serum potassium. Use Caution/Monitor.
- potassium citrate/citric acid
amiloride and potassium citrate/citric acid both increase serum potassium. Use Caution/Monitor.
- potassium iodide
potassium iodide and amiloride both increase serum potassium. Use Caution/Monitor. Potassium salts may increase the hyperkalemic effects of potassium-sparing diuretics.
- propranolol
propranolol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- quinapril
quinapril, amiloride. Mechanism: pharmacodynamic synergism. Use Caution/Monitor. Risk of hyperkalemia.
- quinidine
amiloride, quinidine. Mechanism: unspecified interaction mechanism. Use Caution/Monitor. Increased risk of arrhythmias in pts. with ventricular tachycardia.
- ramipril
ramipril, amiloride. Mechanism: pharmacodynamic synergism. Use Caution/Monitor. Risk of hyperkalemia.
- sacubitril/valsartan
sacubitril/valsartan and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- salicylates (non-asa)
amiloride and salicylates (non-asa) both increase serum potassium. Modify Therapy/Monitor Closely.
- salmeterol
amiloride increases and salmeterol decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- salsalate
amiloride and salsalate both increase serum potassium. Modify Therapy/Monitor Closely.
- sotalol
sotalol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- sparsentan
sparsentan and amiloride both increase serum potassium. Use Caution/Monitor. Monitor serum potassium frequently.
- succinylcholine
amiloride and succinylcholine both increase serum potassium. Modify Therapy/Monitor Closely.
- sulfasalazine
amiloride and sulfasalazine both increase serum potassium. Modify Therapy/Monitor Closely.
- sulindac
amiloride and sulindac both increase serum potassium. Modify Therapy/Monitor Closely.
- tadalafil
tadalafil increases effects of amiloride by pharmacodynamic synergism. Use Caution/Monitor. Risk of hypotension.
- telmisartan
telmisartan and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- temocillin
temocillin increases effects of amiloride by unspecified interaction mechanism. Use Caution/Monitor. Hyperkalemia.
- tenofovir DF
tenofovir DF increases levels of amiloride by decreasing renal clearance. Use Caution/Monitor. Potential for increased toxicity. .
- terbutaline
amiloride increases and terbutaline decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- ticarcillin
ticarcillin increases effects of amiloride by unspecified interaction mechanism. Use Caution/Monitor. Hyperkalemia.
- timolol
timolol and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- tolfenamic acid
amiloride and tolfenamic acid both increase serum potassium. Modify Therapy/Monitor Closely.
- tolmetin
amiloride and tolmetin both increase serum potassium. Modify Therapy/Monitor Closely.
- tolvaptan
amiloride and tolvaptan both increase serum potassium. Modify Therapy/Monitor Closely.
- torsemide
amiloride increases and torsemide decreases serum potassium. Effect of interaction is not clear, use caution. Modify Therapy/Monitor Closely.
- trandolapril
trandolapril, amiloride. Mechanism: pharmacodynamic synergism. Use Caution/Monitor. Risk of hyperkalemia.
- trimethoprim
trimethoprim and amiloride both increase serum potassium. Use Caution/Monitor. Trimethoprim decreases urinary potassium excretion. May cause hyperkalemia, particularly with high doses, renal insufficiency, or when combined with other drugs that cause hyperkalemia.
- valsartan
valsartan and amiloride both increase serum potassium. Modify Therapy/Monitor Closely.
- vandetanib
vandetanib increases levels of amiloride by Other (see comment). Modify Therapy/Monitor Closely. Comment: Vandetanib inhibits the uptake of substrates of organic cation transporter type 2 (OCT2).
- voclosporin
voclosporin and amiloride both increase serum potassium. Use Caution/Monitor.
- xipamide
xipamide increases effects of amiloride by pharmacodynamic synergism. Use Caution/Monitor.
Minor (34)
- agrimony
agrimony increases effects of amiloride by pharmacodynamic synergism. Minor/Significance Unknown.
- amoxicillin
amiloride decreases levels of amoxicillin by inhibition of GI absorption. Applies only to oral form of both agents. Minor/Significance Unknown. Administer each drug at least 2 hours apart from each other; monitor for reduced antibiotic efficacy.
- birch
birch increases effects of amiloride by pharmacodynamic synergism. Minor/Significance Unknown.
- brimonidine
brimonidine increases effects of amiloride by pharmacodynamic synergism. Minor/Significance Unknown.
- cadexomer iodine
cadexomer iodine, amiloride. Mechanism: decreasing renal clearance. Minor/Significance Unknown. Hyperkalemia.
- calcium acetate
amiloride decreases levels of calcium acetate by increasing renal clearance. Minor/Significance Unknown.
- calcium carbonate
amiloride decreases levels of calcium carbonate by increasing renal clearance. Minor/Significance Unknown.
- calcium chloride
amiloride decreases levels of calcium chloride by increasing renal clearance. Minor/Significance Unknown.
- calcium citrate
amiloride decreases levels of calcium citrate by increasing renal clearance. Minor/Significance Unknown.
- calcium gluconate
amiloride decreases levels of calcium gluconate by increasing renal clearance. Minor/Significance Unknown.
- cornsilk
cornsilk increases effects of amiloride by pharmacodynamic synergism. Minor/Significance Unknown.
- entecavir
amiloride, entecavir. Either increases effects of the other by decreasing renal clearance. Minor/Significance Unknown. Coadministration with drugs that reduce renal function or compete for active tubular secretion may increase serum concentrations of either entecavir or the coadministered drug.
- epoprostenol
epoprostenol increases effects of amiloride by pharmacodynamic synergism. Minor/Significance Unknown. Additive hypotensive effects.
- forskolin
forskolin increases effects of amiloride by pharmacodynamic synergism. Minor/Significance Unknown.
- goldenrod
goldenrod increases effects of amiloride by pharmacodynamic synergism. Minor/Significance Unknown.
- iodinated glycerol
iodinated glycerol, amiloride. Mechanism: decreasing renal clearance. Minor/Significance Unknown. Hyperkalemia.
- iodine
iodine, amiloride. Mechanism: decreasing renal clearance. Minor/Significance Unknown. Hyperkalemia.
- isavuconazonium sulfate
isavuconazonium sulfate will increase the level or effect of amiloride by Other (see comment). Minor/Significance Unknown. Isavuconazonium sulfate, an OCT2 inhibitor, may increase the effects or levels of OCT2 substrates.
- magnesium chloride
amiloride increases levels of magnesium chloride by decreasing renal clearance. Minor/Significance Unknown.
- magnesium citrate
amiloride increases levels of magnesium citrate by decreasing renal clearance. Minor/Significance Unknown.
- magnesium hydroxide
amiloride increases levels of magnesium hydroxide by decreasing renal clearance. Minor/Significance Unknown.
- magnesium oxide
amiloride increases levels of magnesium oxide by decreasing renal clearance. Minor/Significance Unknown.
- magnesium sulfate
amiloride increases levels of magnesium sulfate by decreasing renal clearance. Minor/Significance Unknown.
- maitake
maitake increases effects of amiloride by pharmacodynamic synergism. Minor/Significance Unknown.
- minoxidil
amiloride increases effects of minoxidil by pharmacodynamic synergism. Minor/Significance Unknown.
- octacosanol
octacosanol increases effects of amiloride by pharmacodynamic synergism. Minor/Significance Unknown.
- reishi
reishi increases effects of amiloride by pharmacodynamic synergism. Minor/Significance Unknown.
- shepherd's purse
shepherd's purse, amiloride. Other (see comment). Minor/Significance Unknown. Comment: Theoretically, shepherd's purse may interfere with BP control.
- sulfadiazine
amiloride increases levels of sulfadiazine by unspecified interaction mechanism. Minor/Significance Unknown.
- sulfamethoxazole
amiloride, sulfamethoxazole. Mechanism: unspecified interaction mechanism. Minor/Significance Unknown. Hyperkalemia.
amiloride increases levels of sulfamethoxazole by unspecified interaction mechanism. Minor/Significance Unknown. - sulfisoxazole
amiloride increases levels of sulfisoxazole by unspecified interaction mechanism. Minor/Significance Unknown.
- tizanidine
tizanidine increases effects of amiloride by pharmacodynamic synergism. Minor/Significance Unknown. Risk of hypotension.
- treprostinil
treprostinil increases effects of amiloride by pharmacodynamic synergism. Minor/Significance Unknown.
- trimethoprim
amiloride, trimethoprim. Mechanism: unspecified interaction mechanism. Minor/Significance Unknown. Hyperkalemia.
Adverse Effects
1-10%
Hyperkalemia (10%)
Anorexia (3-8%)
Diarrhea (3-8%)
Headache (3-8%)
Nausea (3-8%)
Vomiting (3-8%)
Abdominal pain (<3%)
Appetite changes (<3%)
Constipation (<3%)
Cough (<3%)
Dizziness (<3%)
Dyspnea (<3%)
Encephalopathy (<3%)
Fatigue (<3%)
Gas pain (<3%)
Impotence (<3%)
Muscle cramps (<3%)
Weakness (<3%)
Warnings
Black Box Warnings
Like other potassium-conserving agents, amiloride may cause hyperkalemia (serum potassium levels greater than 5.5 mEq/L), which, if not corrected, is potentially fatal.
Hyperkalemia occurs commonly (about 10%) when amiloride is used without a kaliuretic diuretic.
This incidence is greater in patients with renal impairment, diabetes mellitus (with or without recognized renal insufficiency), and in elderly persons.
When amiloride is used concomitantly with a thiazide diuretic in patients without these complications, the risk of hyperkalemia is reduced to about 1-2%. Monitoring serum potassium levels carefully in any patient receiving amiloride is essential, particularly when it is first introduced, at the time of diuretic dosage adjustments, and during any illness that could affect renal function.
Contraindications
Hypersensitivity to amiloride
Hyperkalemia (K+ >5.5 mEq/L [5.5 mmol/L])
Concomitant use with K+-sparing diuretic, or K supplementation
Impaired renal function (Scr >1.5 mg/dL [132.6 umol/L], or BUN >30 mg/dL [10.7 mmol/L]) diabetes
Cautions
Hyperkalemia
- The risk of hyperkalemia may be increased when potassium-conserving agents, including amiloride hydrochloride are administered concomitantly with an angiotensin-converting enzyme inhibitor, an angiotensin II receptor antagonist, cyclosporine or tacrolimus
- Warning signs or symptoms of hyperkalemia include paresthesias, muscular weakness, fatigue, flaccid paralysis of the extremities, bradycardia, shock, and ECG abnormalities; monitoring of the serum potassium level is essential because mild hyperkalemia is not usually associated with an abnormal ECG
- When abnormal, the ECG in hyperkalemia is characterized primarily by tall, peaked T waves or elevations from previous tracings; there may also be lowering of the R wave and increased depth of the S wave, widening and even disappearance of the P wave, progressive widening of the QRS complex, prolongation of the PR interval, and ST depression
- If hyperkalemia occurs the drug should be discontinued immediately; if serum potassium level exceeds 6.5 mEq per liter, active measures should be taken to reduce it; such measures include the intravenous administration of sodium bicarbonate solution or oral or parenteral glucose with a rapid-acting insulin preparation; if needed, a cation exchange resin such as sodium polystyrene sulfonate may be given orally or by enema; patients with persistent hyperkalemia may require dialysis
Diabetes Mellitus
- In diabetic patients, hyperkalemia has been reported with the use of all potassium-conserving diuretics, including amiloride hydrochloride, even in patients without evidence of diabetic nephropathy; therefore, amiloride should be avoided, if possible, in diabetic patients and, if it is used, serum electrolytes and renal function must be monitored frequently; amiloride should be discontinued at least 3 days before glucose tolerance testing
Metabolic or Respiratory Acidosis
- Antikaliuretic therapy should be instituted only with caution in severely ill patients in whom respiratory or metabolic acidosis may occur, such as patients with cardiopulmonary disease or poorly controlled diabetes
- If therapy is administered to these patients, frequent monitoring of acid-base balance necessary; shifts in acid-base balance alter ratio of extracellular/intracellular potassium, and development of acidosis may be associated with rapid increases in serum potassium levels
- In patients with renal disease, diuretics may precipitate azotemia; cumulative effects of the components of amiloride hydrochloride may develop in patients with impaired renal function; if renal impairment becomes evident discontinue therapy
Drug interaction overview
- In some patients, the administration of a non-steroidal anti-inflammatory agent can reduce the diuretic, natriuretic, and antihypertensive effects of loop and potassium-sparing diuretics
- When amiloride and non-steroidal anti-inflammatory agents are used concomitantly, the patient should be observed closely to determine if the desired effect of the diuretic is obtained
- Since indomethacin and potassium-sparing diuretics, including this product, may each be associated with increased serum potassium levels, the potential effects on potassium kinetics and renal function should be considered when these agents are administered concurrently
Pregnancy & Lactation
Pregnancy
Teratogenicity studies in rabbits and mice given 20 and 25 times maximum human dose, respectively, revealed no evidence of harm to fetus, although studies showed that the drug crossed the placenta in modest amounts; reproduction studies in rats at 20 times the expected maximum daily dose for humans showed no evidence of impaired fertility
At approximately 5 or more times the expected maximum daily dose for humans, some toxicity was seen in adult rats and rabbits and a decrease in rat pup growth and survival occurred
Lactation
Studies in rats have shown that amiloride is excreted in milk in concentrations higher than those found in blood, but it is not known whether amiloride hydrochloride is excreted in human milk
Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother
Pregnancy Categories
A: Generally acceptable. Controlled studies in pregnant women show no evidence of fetal risk.
B: May be acceptable. Either animal studies show no risk but human studies not available or animal studies showed minor risks and human studies done and showed no risk. C: Use with caution if benefits outweigh risks. Animal studies show risk and human studies not available or neither animal nor human studies done. D: Use in LIFE-THREATENING emergencies when no safer drug available. Positive evidence of human fetal risk. X: Do not use in pregnancy. Risks involved outweigh potential benefits. Safer alternatives exist. NA: Information not available.Pharmacology
Mechanism of Action
Exerts its potassium sparing effect through the inhibition of sodium reabsorption at the distal convoluted tubule, cortical collecting tubule and collecting duct; this decreases the net negative potential of the tubular lumen and reduces both potassium and hydrogen secretion and their subsequent excretion. This mechanism accounts in large part for the potassium sparing action of amiloride.
Inhibits Na/K-ATPase, decreases Ca++, Mg++ and hydrogen excretion
Pharmacokinetics
Half-Life: 6-9 hr
Duration: 24 hr
Onset: initial effect: 2-3 hr, max effect: 6-10 hr
Peak Plasma Time: 3-4 hr
Bioavailability: 30-90%
Protein Bound: 23%
Vd: 350-380 L
Metabolism: NOT metabolized in the liver; no active metabolites
Excretion
- Urine: 50%
- Feces: 40-50%
Images
| BRAND | FORM. | UNIT PRICE | PILL IMAGE |
|---|---|---|---|
| amiloride oral - | 5 mg tablet |  | |
| amiloride oral - | 5 mg tablet |  | |
| amiloride oral - | 5 mg tablet | 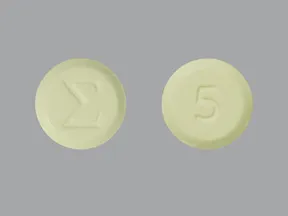 |
Copyright © 2010 First DataBank, Inc.
Patient Handout
amiloride oral
AMILORIDE - ORAL
(a-MIL-oh-ride)
COMMON BRAND NAME(S): Midamor
WARNING: This medication can cause high potassium levels (hyperkalemia). This effect is more likely to occur in older adults and in patients with kidney disease, diabetes, or a serious illness. Potassium levels must be closely monitored on a regular basis while taking this medication. If not treated, very high potassium levels can sometimes be fatal. Tell your doctor right away if you develop any symptoms of high potassium levels, including muscle weakness, slow/irregular heartbeat, numb/tingling skin.
USES: Amiloride is used with other "water pills"/diuretics (such as furosemide, thiazide diuretics like hydrochlorothiazide) to treat high blood pressure (hypertension), heart failure, or extra fluid in the body (edema). Amiloride also helps to treat or prevent low blood potassium levels caused by the other diuretics. Lowering high blood pressure helps prevent strokes, heart attacks, and kidney problems.Amiloride is called a "water pill" (diuretic) and causes your body to get rid of extra salt and water while also preventing the kidneys from getting rid of too much potassium.
HOW TO USE: Take this medication by mouth with food as directed by your doctor, usually once daily. The dosage is based on your medical condition and response to treatment.If you take this drug too close to bedtime, you may need to wake up to urinate. It is best to take this medication at least 4 hours before your bedtime. If you have any questions about how and when to take this medication, ask your doctor or pharmacist.Use this medication regularly in order to get the most benefit from it. To help you remember, take it at the same time each day. Keep taking this medication even if you feel well. Most people with high blood pressure do not feel sick.Tell your doctor if your condition does not get better or if it gets worse (for example, your blood pressure readings remain high or increase).
SIDE EFFECTS: See also Warning section.Headache, dizziness, nausea, vomiting, loss of appetite, stomach/abdominal pain, gas, or diarrhea may occur. If any of these effects last or get worse, tell your doctor or pharmacist promptly.Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.When given with other diuretics, this medication may cause dehydration and electrolyte imbalance. Tell your doctor right away if you have any symptoms of dehydration or electrolyte imbalance, including unusual dry mouth/thirst, muscle cramps/weakness, slow/fast/irregular heartbeat, or confusion.Tell your doctor right away if you have any serious side effects, including: fainting, signs of liver problems (such as nausea/vomiting that doesn't stop, yellowing eyes/skin, dark urine), signs of kidney problems (such as change in the amount of urine).A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.In the US -Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
PRECAUTIONS: See Also Warning section.Before taking amiloride, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.Before using this medication, tell your doctor or pharmacist your medical history, especially of: untreated salt/mineral imbalance (such as high potassium, low sodium level), kidney disease, liver disease, diabetes, dehydration.Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).This drug may make you dizzy. Alcohol or marijuana (cannabis) can make you more dizzy. Do not drive, use machinery, or do anything that needs alertness until you can do it safely. Limit alcoholic beverages. Talk to your doctor if you are using marijuana (cannabis).Severe sweating, diarrhea, or vomiting may cause dehydration and cause you to feel lightheaded. Tell your doctor if you have severe diarrhea or vomiting. To prevent dehydration, drink plenty of fluids unless your doctor tells you not to.This drug may increase the potassium levels in your blood. Limit foods high in potassium such as bananas and orange juice. Consult your doctor or pharmacist before using any products containing potassium (such as potassium supplements, salt substitutes).During pregnancy, this medication should be used only when clearly needed. Discuss the risks and benefits with your doctor.It is unknown if this drug passes into breast milk. Consult your doctor before breastfeeding.
DRUG INTERACTIONS: See also Precautions section.Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.Some products that may interact with this drug are: lithium, sodium phosphate products, other products that may increase potassium levels (such as eplerenone, tacrolimus, cyclosporine, birth control pills containing drospirenone, potassium-sparing diuretics like spironolactone/triamterene, ACE inhibitors like benazepril/captopril, angiotensin receptor antagonists like losartan/valsartan).Some products have ingredients that could raise your blood pressure or worsen your heart failure. Tell your pharmacist what products you are using, and ask how to use them safely (especially cough-and-cold products, diet aids, or NSAIDs such as ibuprofen/naproxen).This medication may interfere with certain lab tests (such as glucose tolerance testing), possibly causing false test results. Make sure lab personnel and all your doctors know you use this drug.
OVERDOSE: If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center. Symptoms of overdose may include: severe dizziness, fainting, slow/irregular heartbeat.
NOTES: Do not share this medication with others.Lifestyle changes such as stress reduction programs, exercise, and dietary changes may increase the effectiveness of this medicine. Talk to your doctor or pharmacist about lifestyle changes that might benefit you.Lab and/or medical tests (including kidney function, blood mineral levels such as potassium) should be done while you are taking this medication. Keep all medical and lab appointments. Consult your doctor for more details.Check your blood pressure regularly while taking this medication, especially when you first start this drug or when your dose is changed. Learn how to monitor your own blood pressure at home, and share the results with your doctor.
MISSED DOSE: If you miss a dose, take it as soon as you remember. If it is near the time of the next dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
STORAGE: Store at room temperature away from light and moisture. Protect from freezing. Do not store in the bathroom. Keep all medications away from children and pets.Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Information last revised March 2024. Copyright(c) 2024 First Databank, Inc.
IMPORTANT: HOW TO USE THIS INFORMATION: This is a summary and does NOT have all possible information about this product. This information does not assure that this product is safe, effective, or appropriate for you. This information is not individual medical advice and does not substitute for the advice of your health care professional. Always ask your health care professional for complete information about this product and your specific health needs.
Formulary
Adding plans allows you to compare formulary status to other drugs in the same class.
To view formulary information first create a list of plans. Your list will be saved and can be edited at any time.
Adding plans allows you to:
- View the formulary and any restrictions for each plan.
- Manage and view all your plans together – even plans in different states.
- Compare formulary status to other drugs in the same class.
- Access your plan list on any device – mobile or desktop.


